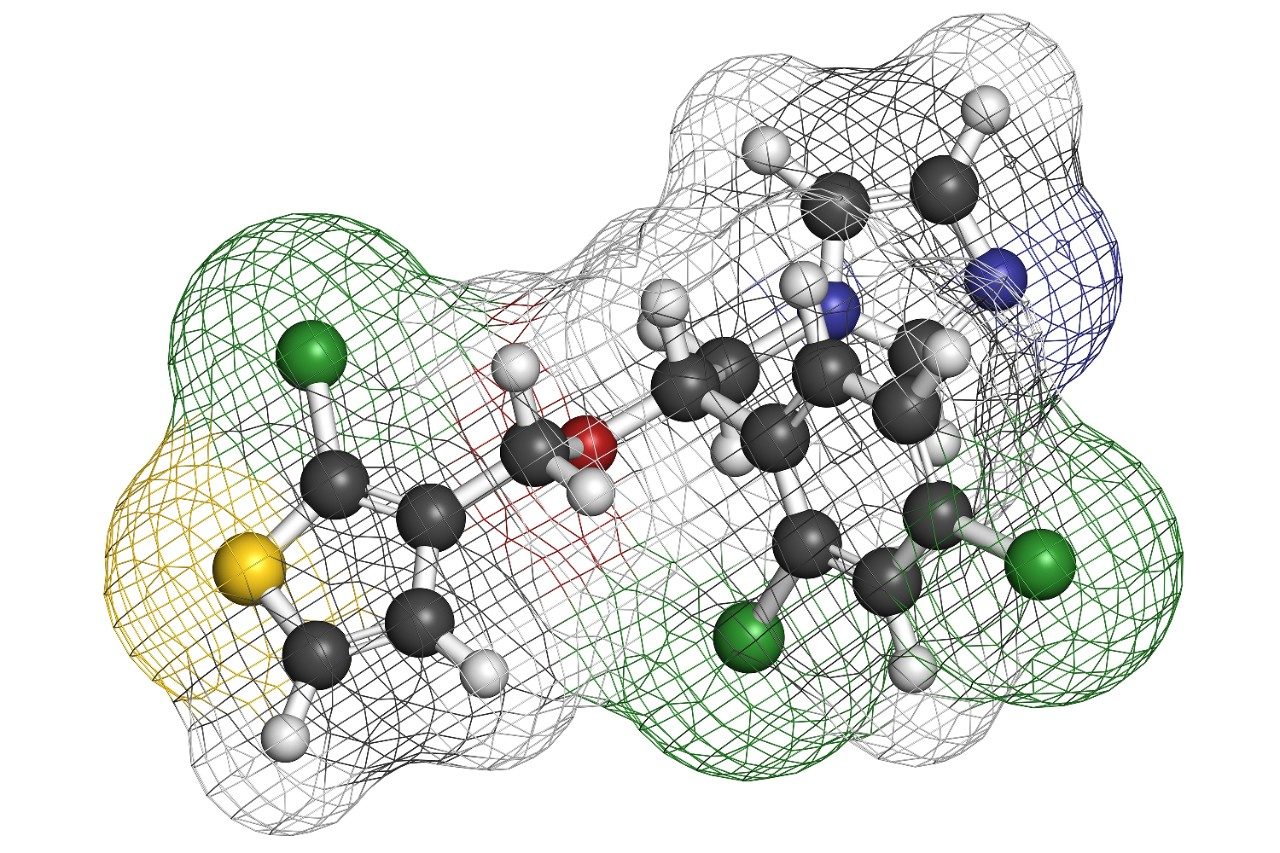
Tioconazole is produced generically and routinely analyzed worldwide by pharmaceutical manufacturers. In this application, the separation of tioconazole and tioconazole related compounds was demonstrated using the USP organic impurities method specified in the USP monograph on several different column dimensions. The Alliance HPLC System is used for its robust and reproducible performance in high throughput environments.
Organic impurities in generic drugs are routinely analyzed by pharmaceutical manufacturers worldwide. Performing organic impurities analyses with older instrumentation and column technology can be time-consuming and costly, as these methods can be very lengthy and require large amounts of solvent. However, by using more modern chromatographic tools, organic impurities assays can become more efficient due to significant improvements in both instrumentation and column technology. eXtended Performance (XP) columns are 2.5 μm particle size columns designed for use on both HPLC and UPLC instrumentation. These columns are ideal for modernizing USP methods as they allow chromatographers to realize the benefit of smaller particle sizes, while operating according to the USP Chromatography Chapter <621> guidelines.1
Tioconazole is an imidazole antifungal compound used in the treatment of yeast infections. The method that was transferred is the organic impurities analysis of tioconazole.2 Organic impurity methods are used to determine the presence and quantity of impurities in a sample. The USP method was scaled from the original column dimensions to XP columns on an Alliance HPLC System. The integrated fluidic design and efficient solvent management features of the Alliance HPLC System make it robust for reliable quality data generation. Updating the current USP method using XP columns on an HPLC instrument can reduce run times, thereby increasing sample throughput in a routine analytical laboratory.
|
Mobile phase A: |
44:40:28 Acetonitrile/ methanol/water with 2 mL ammonium hydroxide |
|
Separation mode: |
Isocratic |
|
Detector: |
2998 photodiode array (PDA) |
|
UV wavelength: |
219 nm |
|
Column (L1): |
XSelect CSH C18 4.6 x 250 mm, 5 μm; XSelect CSH XP C18 4.6 x 150 mm, 2.5 μm; XSelect CSH XP C18 4.6 x 100 mm, 2.5 μm |
|
Column temp.: |
25 °C |
|
Needle wash: |
95:5 ACN/water |
|
Seal wash: |
50:50 MeOH/water |
|
Flow rate: |
1.0 mL/min |
|
Injection volume: |
25 μL (250 mm column), 12 μL (150 mm column), 8 μL (100 mm column) |
|
Data management: |
Empower 3 Software |
The tioconazole sample was prepared in 100% methanol to the concentrations described in Table 1. The sample was then transferred to a TruView Maximum Recovery Vial for injection.
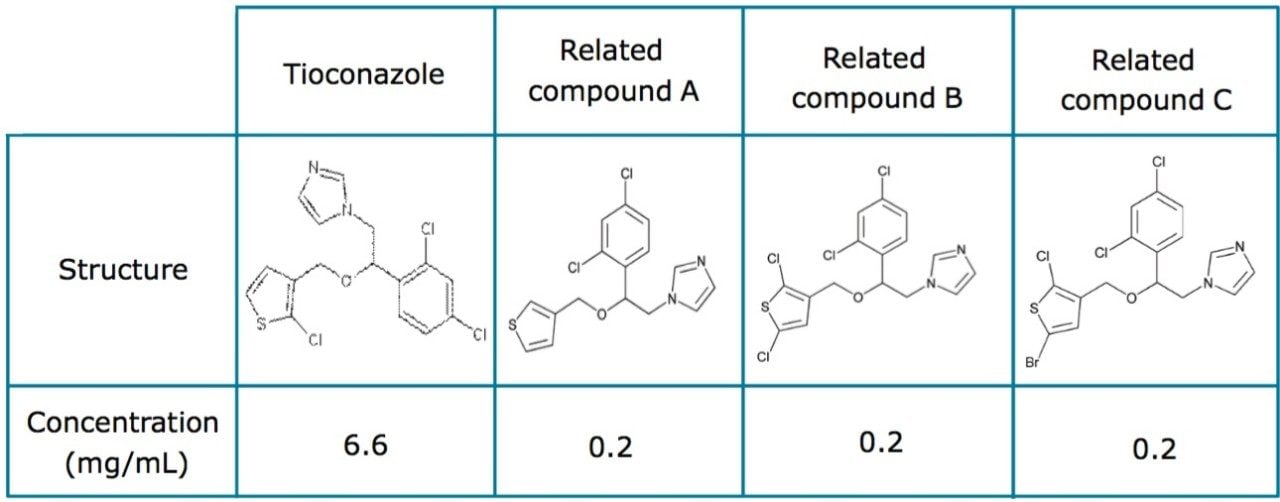
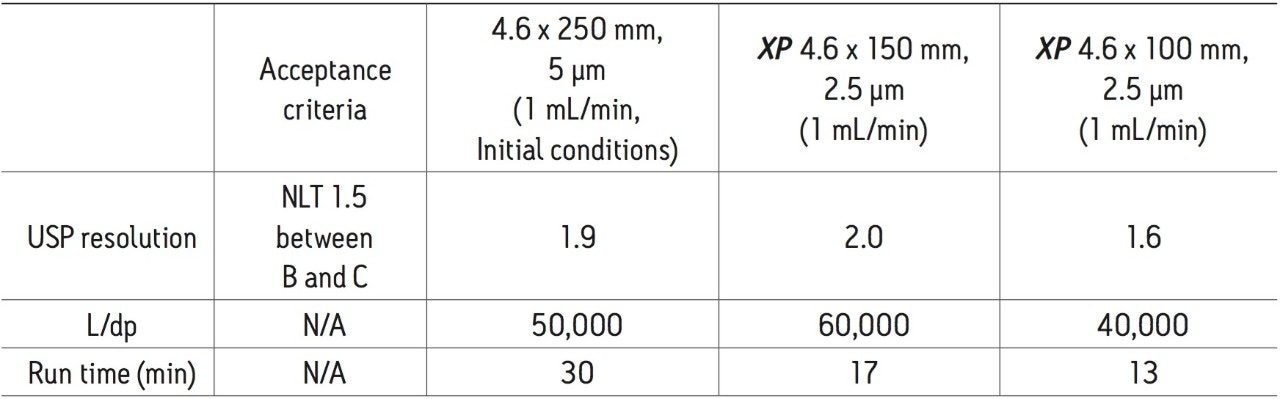
Tioconazole is produced generically and routinely analyzed worldwide by pharmaceutical manufacturers. In this application, the separation of tioconazole and tioconazole related compounds A, B, and C was demonstrated using the USP organic impurities method specified in the USP monograph on several different column dimensions. The Alliance HPLC System is used for its robust and reproducible performance in high throughput environments. The simplified fluidic path and integrated sample and solvent management result in pulse-free solvent flow, reduced system dispersion, and generate high quality reliable data. Tioconazole related compounds A, B, and C were used as low level impurity standards, as tioconazole impurities were not readily available. Organic impurity methods listed in the USP are used to analyze complex sample formulations. The well-resolved separation of multiple components in the samples often requires the use of longer column dimensions. The use of longer columns with larger particle sizes (≥3.5 μm) results in long run times and large amounts of solvent consumption. For example, the original USP organic impurities analysis of tioconazole requires a 4.6 x 250 mm, 5 μm column and 30 mL of solvent per sample per analysis with the separation taking thirty minutes to complete. However, using eXtended Performance (XP) 2.5 μm particle columns, run times may be reduced while meeting assay requirements. With shorter run times, throughput can increase with less solvent used per analysis, leading to overall cost savings. The current USP <621> Chromatography chapter provides allowable method changes that include ±70% change in column length, -50% change in particle size, and ±50% change in flow rate.1 These guidelines were followed throughout the method transfers demonstrated here. A USP resolution of 1.5 between related compounds B and C was used as a requirement in this application to demonstrate that this critical pair can be consistently resolved as the method is transferred across different column dimensions.
The organic impurities method for the analysis of tioconazole requires the use of an L1 USP designated column with the listed column for this separation a LiChrosorb RP-18.2 Using the Waters Reversed Phase Column Selectivity Chart, the more modern XSelect CSH C18 stationary phase was chosen. The XSelect CSH C18 column was chosen because of its similarity to the listed column and its ability to provide full scalability of dimensions and particle sizes between HPLC and UPLC instrumentation. The USP method for this separation was first run using an Alliance HPLC System with an XSelect CSH C18 4.6 x 250 mm, 5 μm column with a flow rate of 1.0 mL/min. The acceptance criteria for this separation were met, as shown in Table 2. The total run time for this separation was 30 minutes, which poses challenges in both time and financial management in high throughput environments where samples are continuously analyzed. Using the original USP method, an eight-hour work shift would result in only 16 samples being analyzed with 480 mL of solvent used. By using XP columns, up to 80 samples can be analyzed in the same eight-hour shift using only 240 mL of solvent, thus significantly increasing throughput and reducing operating costs.
The versatility of modernizing the compendial method using XP 2.5 μm columns across different column configurations on the Alliance HPLC System, while remaining within USP <621> guidelines, is shown in Figure 1.
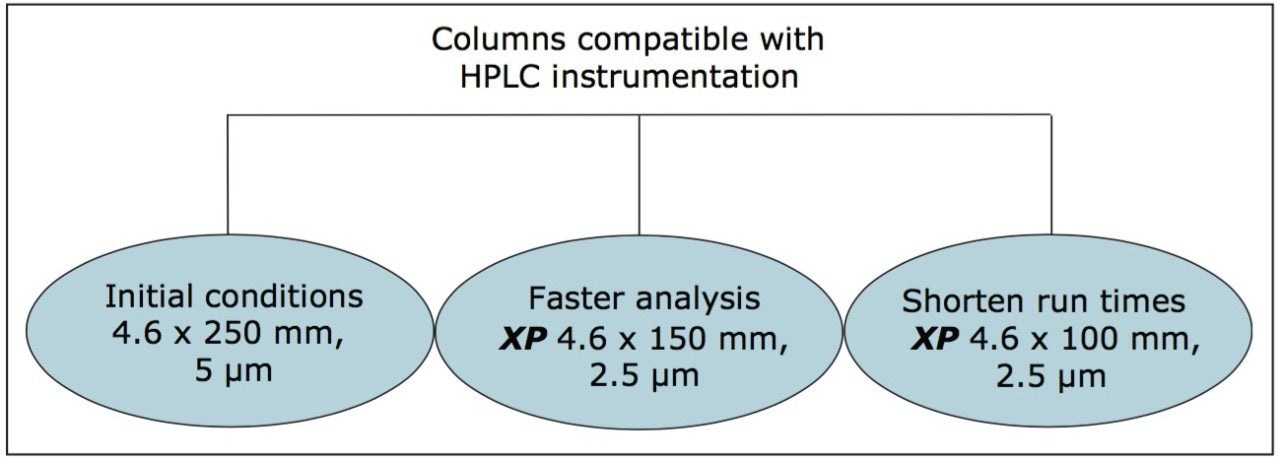
The compendial method was first transferred from the original 4.6 x 250 mm, 5 μm column to an XP 4.6 x 150 mm, 2.5 μm column to demonstrate that shorter run times can be achieved by using smaller particle sizes. Adopting smaller particle sizes can also lead to an increase in resolving power, measured by the column length to particle size ratio (L/dp). In this case, the L/dp increases from 50,000 (initial conditions) to 60,000 when moving to an XP 4.6 x 150 mm column. According to the ACQUITY UPLC Column Calculator, the properly scaled flow rate for this column is 2.0 mL/min.3 However, that flow rate does not comply with USP <621> guidelines. A flow rate of 1.0 mL/min was used to remain within USP guidelines. The separation of tioconazole and its related compounds on the original column compared to the XP 4.6 x 150 mm column are shown in Figures 2A and 2B. The XP 4.6 x 150 mm column shows a 43% reduction in run time, along with a 5% increase in resolution, as shown in Table 2.
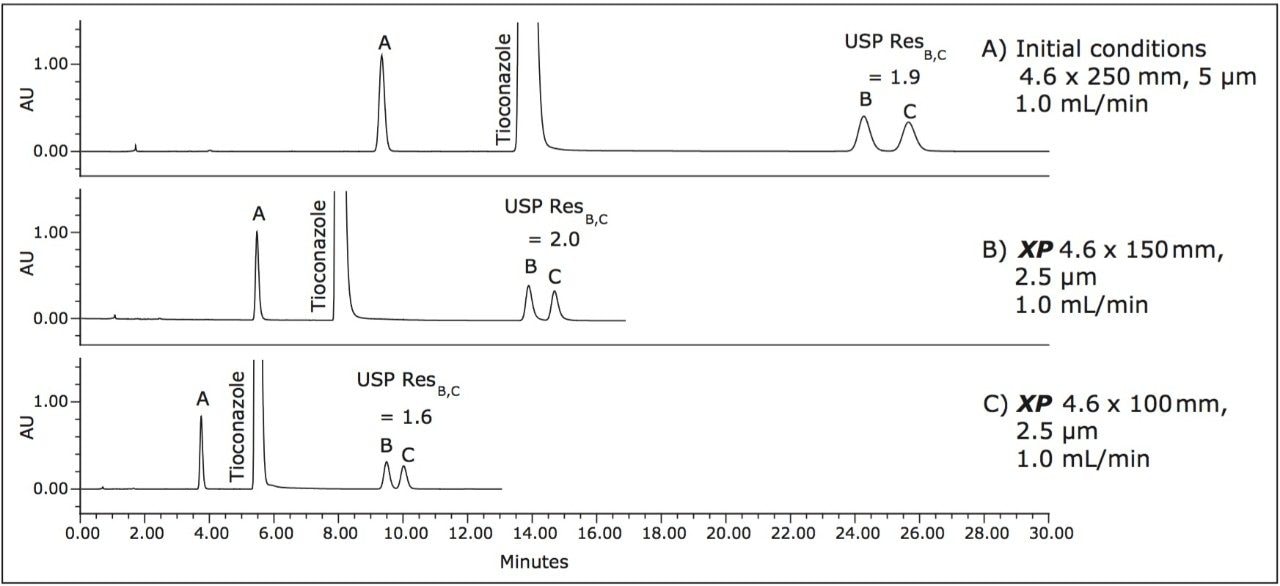
Next, the separation was performed using a shorter XP 4.6 x 100 mm, 2.5 μm column to demonstrate a faster separation while maintaining acceptable resolution. The reduced run times especially benefit organic impurity methods as these methods generally have longer run times than other methods due to the added complexity of the separation. It is important to note that moving to a shorter column with lower resolving power (L/dp 40,000) may not always be an option. For example, cases exist where closely eluting excipients and impurities may require the resolving power of the original separation. Figure 2C shows the separation using the XP 4.6 x 100 mm, 2.5 μm column resulting in a 57% reduction in run time compared to the initial conditions while meeting all of the acceptance criteria, as shown in Table 2. In this case, the reduction in L/dp from 50,000 (initial conditions) to 40,000 resulted in a 15% drop in resolution between related compounds B and C; however, the resolution may still be adequate, depending on the complexity of the original separation.
When performing the often lengthy and costly analysis of organic impurities, the use of eXtended Performance [XP] 2.5 μm columns on existing HPLC systems can significantly reduce run times and solvent usage by up to 57%, compared to the original compendial USP procedure. The availability of XP columns, capable of being run on both HPLC and UPLC instrumentation, allows USP methods to be updated while following the current USP <621> guidelines. In routine analytical laboratories, modernizing USP methods using columns with smaller particle sizes can result in significant time and operating cost savings.
720004554, January 2013