In this study, we demonstrate detection of octreotide in human plasma at very low concentrations using a Waters ACQUITY UPLC System coupled with a Xevo TQ-S tandem quadrupole mass spectrometer. When compared to an HPLC, UPLC helps a user achieve faster separations with lower consumption of mobile phase. In addition, UPLC offers short run times with low dispersion resulting in better separation of signals from unwanted signals from plasma, phospholipids, and other endogenous materials.
A unique optimization of this Waters system for bioanalysis, from sample preparation to UPLC chromatography to tandem quadrupole MS, enables the determination and quantification of octreotide at the 5 pg/mL level.
Octreotide is an octapeptide (Figure 1) that mimics natural somatostatin pharmacologically, and compared to natural hormone is a more potent inhibitor of growth hormone, glucagon, and insulin. Octreotide has been used to treat malignant bowel obstruction. Octreotide is also used in the treatment of acromegaly, diarrhea, and flushing episodes associated with carcinoid syndrome. In addition, octreotide has been used with varying degrees of success in infants to decrease insulin hypersecretion, and investigated for patients with pain from chronic pancreatitis.
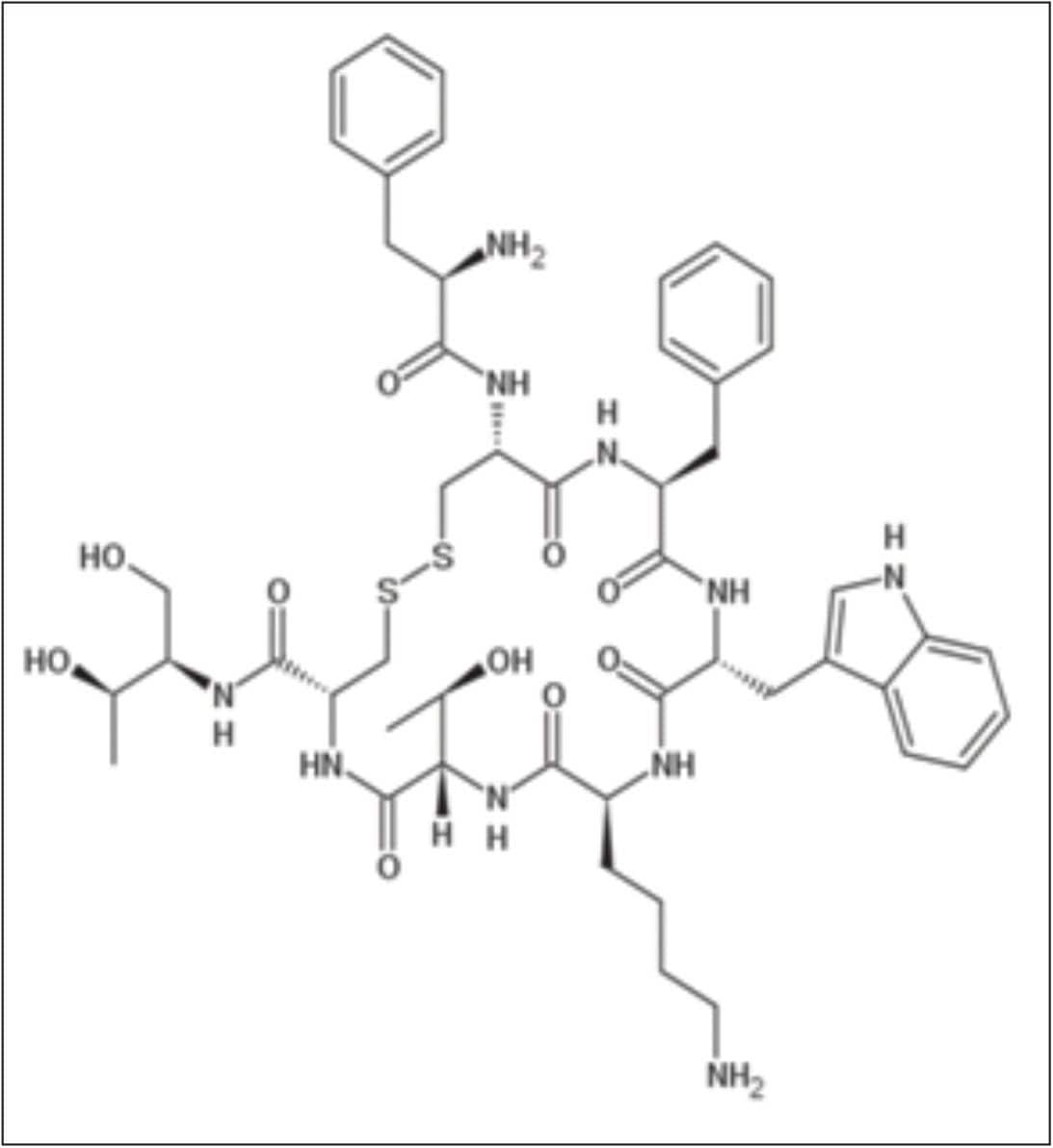
Despite the wide therapeutic use of octreotide, it is a challenge to identify the fate of this drug and its metabolites in plasma because of a lack of analytical instruments and technologies that can monitor drug concentrations at very low pg/mL levels. Although an LC-MS/MS method has been previously reported1 for the quantitative analysis of octreotide in human plasma, the sample preparation procedure included a manual protein precipitation step followed by liquid-liquid extraction step. Such steps are not well suited for high-throughput applications.
In this study, we demonstrate detection of octreotide in human plasma at very low concentrations using a Waters ACQUITY UPLC System coupled with a Xevo TQ-S tandem quadrupole mass spectrometer. When compared to an HPLC, UPLC helps a user achieve faster separations with lower consumption of mobile phase. In addition, UPLC offers short run times with low dispersion resulting in better separation of signals from unwanted signals from plasma, phospholipids, and other endogenous materials.
This study will demonstrate the robustness of the method that was developed to analyze octreotide for a range of 5 to 640 pg/mL.
All the dilutions for octreotide were made in 50% (v/v) methanol in water to obtained required spiking solutions. The plasma was then spiked with diluted octreotide solutions and calibration standards ranging from 5 to 640 pg/mL were prepared. Leuprolide was used as an internal standard (IS) at a concentration of 2 ng/mL, also prepared with 50% (v/v) methanol in water. The samples were isolated by solid phase extraction (SPE) utilizing a Waters Oasis WCX cartridge. A 500 μL aliquot of plasma with 50 μL of IS was diluted with an aqueous acidic solution and loaded onto the SPE cartridge previously condition with organic solvent and water. The plasma solution was then washed with a basic solution, eluted in solvent, evaporated to dryness and reconstituted in mobile phase for analysis by LC-MS/MS.
|
LC system: |
ACQUITY UPLC System equipped with a Binary Solvent Manager, Column Manager, and Sample Manager |
|
LC column: |
ACQUITY UPLC BEH 300 C18, 2.1 x 100 mm, 1.7 μm |
|
Gradient: |
Reversed-phase chromatography with an acidic aqueous buffer solution and acetonitrile as the organic modifier |
|
Elution: |
20-80% organic gradient over 3.25 min |
|
LC gradient run: |
5.0 min starting with 90% acid solution and 10% acetonitrile |
|
Column temp.: |
50 °C |
|
MS system: |
Xevo TQ-S |
|
MS mode: |
Positive ion electrospray MS/MS |
|
MS transition: |
510.54 ⇒ 120.10 |
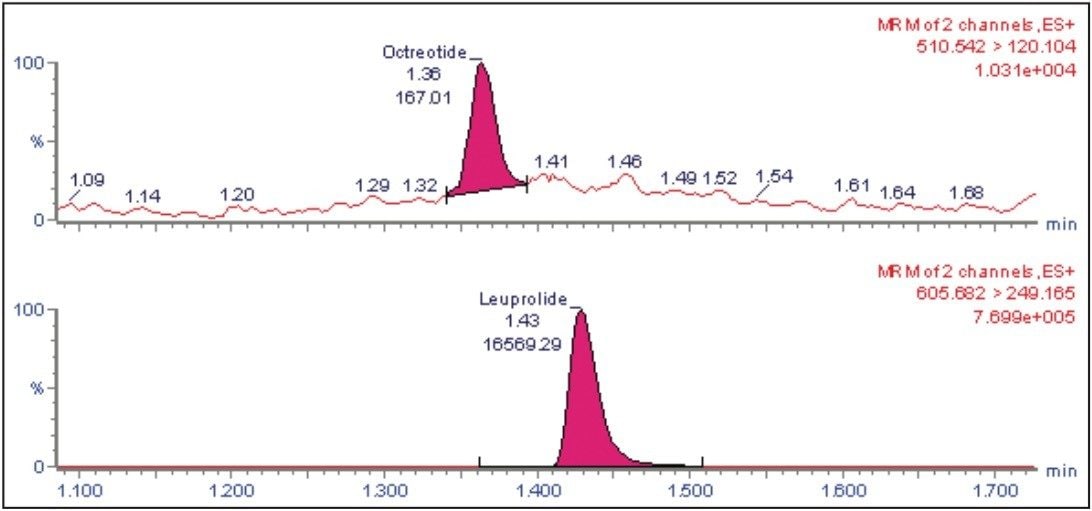
Octreotide is a cyclic peptide with molecular weight of 1019.24 while its IS, leuprolide, is a single, non-glycosylated polypeptide chain with a molecular weight of 1209.4. The octreotide analyte eluted with a retention time of 1.39 min. The chromatogram in Figure 2 shows excellent symmetrical peak shape and resolution from endogenous interferences. In addition, all the six chromatograms at the 5 pg/mL lower level of quantitation (LLOQ) level for octreotide showed signal-to-noise ratio greater than 20 (Figure 2).
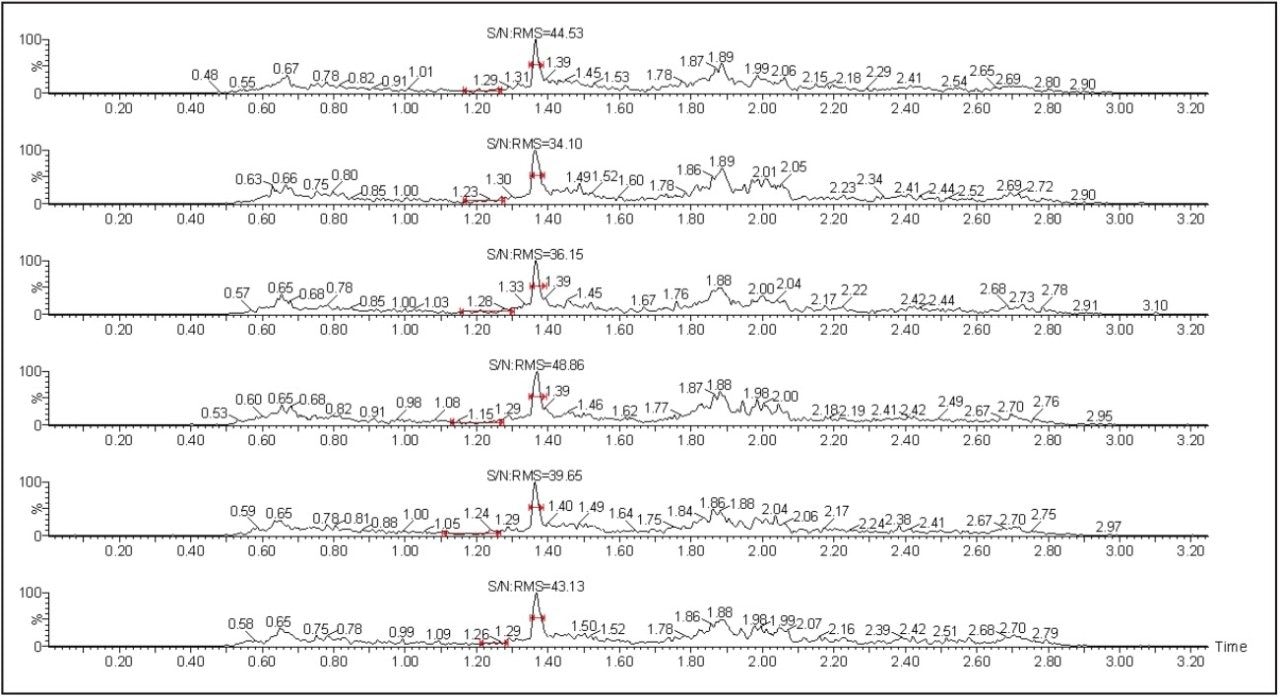
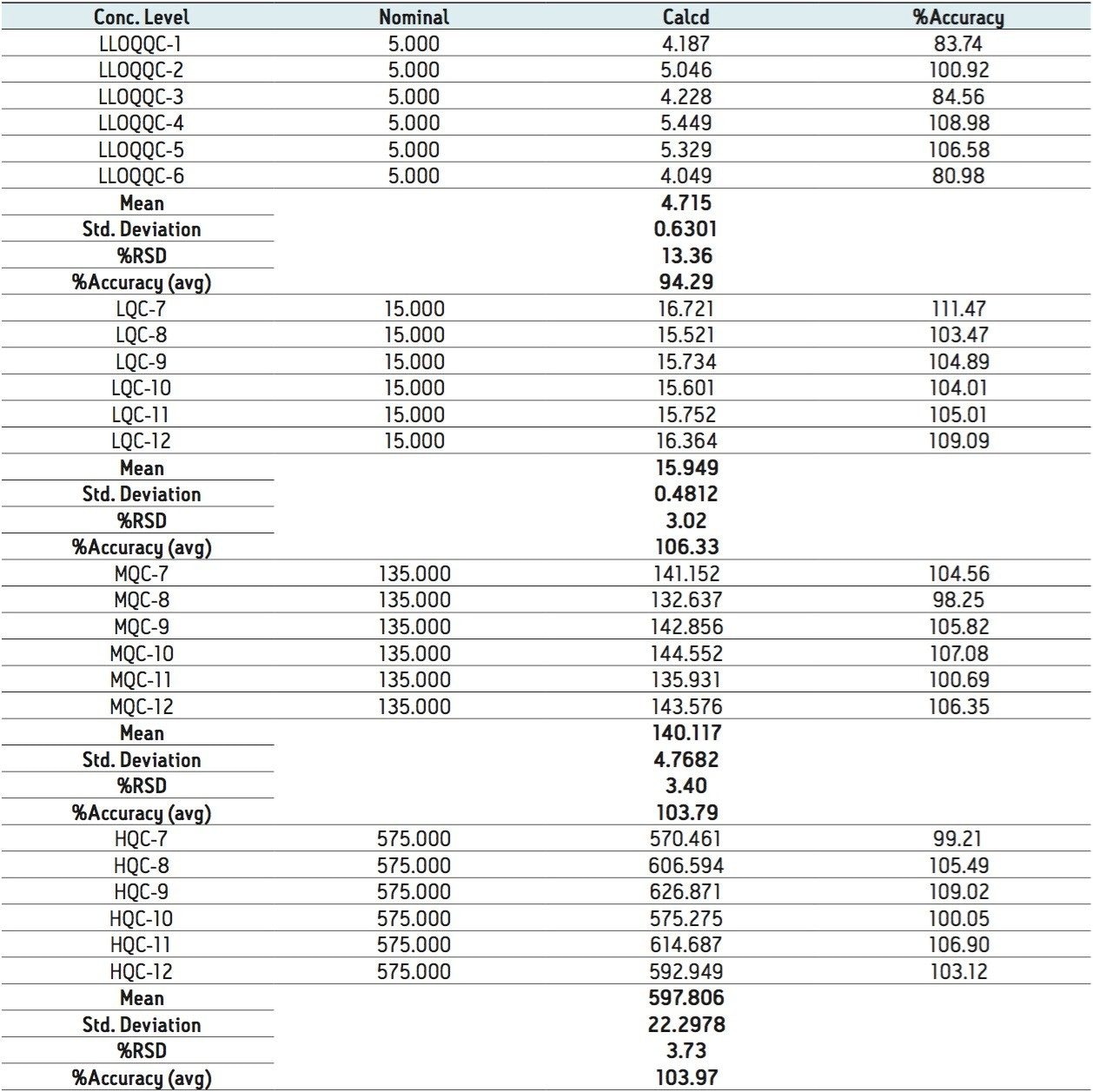
The quality of the peak shape and resolution can be attributed to an optimized combination of the ACQUITY UPLC System and its ACQUITY UPLC BEH 300 C18 Column. Also, the results showed excellent reproducibility at the LLOQ levels (Table 1), which provides a unique ability to quantify octreotide at the LLOQ concentration.
The high quality data obtained for octreotide at the LLOQ level can also be attributed to the high sensitivity detection capabilities provided by the Xevo TQ-S System. The co-joined, off-axis StepWave ion guide in the Xevo TQ-S provides superior levels of sensitivity while maintaining the robustness and cleanliness of the source and ion optics. This enables the Xevo TQ-S to increase the ion flux entering the mass spectrometer, resulting in the highest levels of sensitivity. The assay reported in this study was demonstrated to be linear over the range of 5 to 640 pg/mL in both organic solvents and human plasma samples.
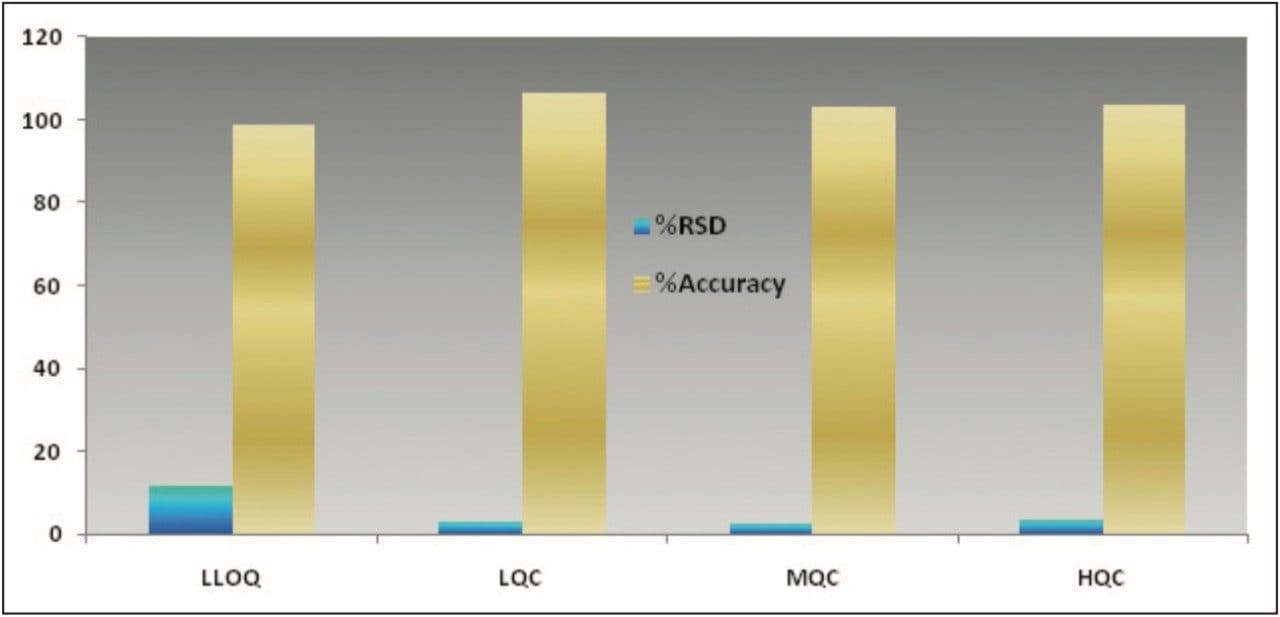
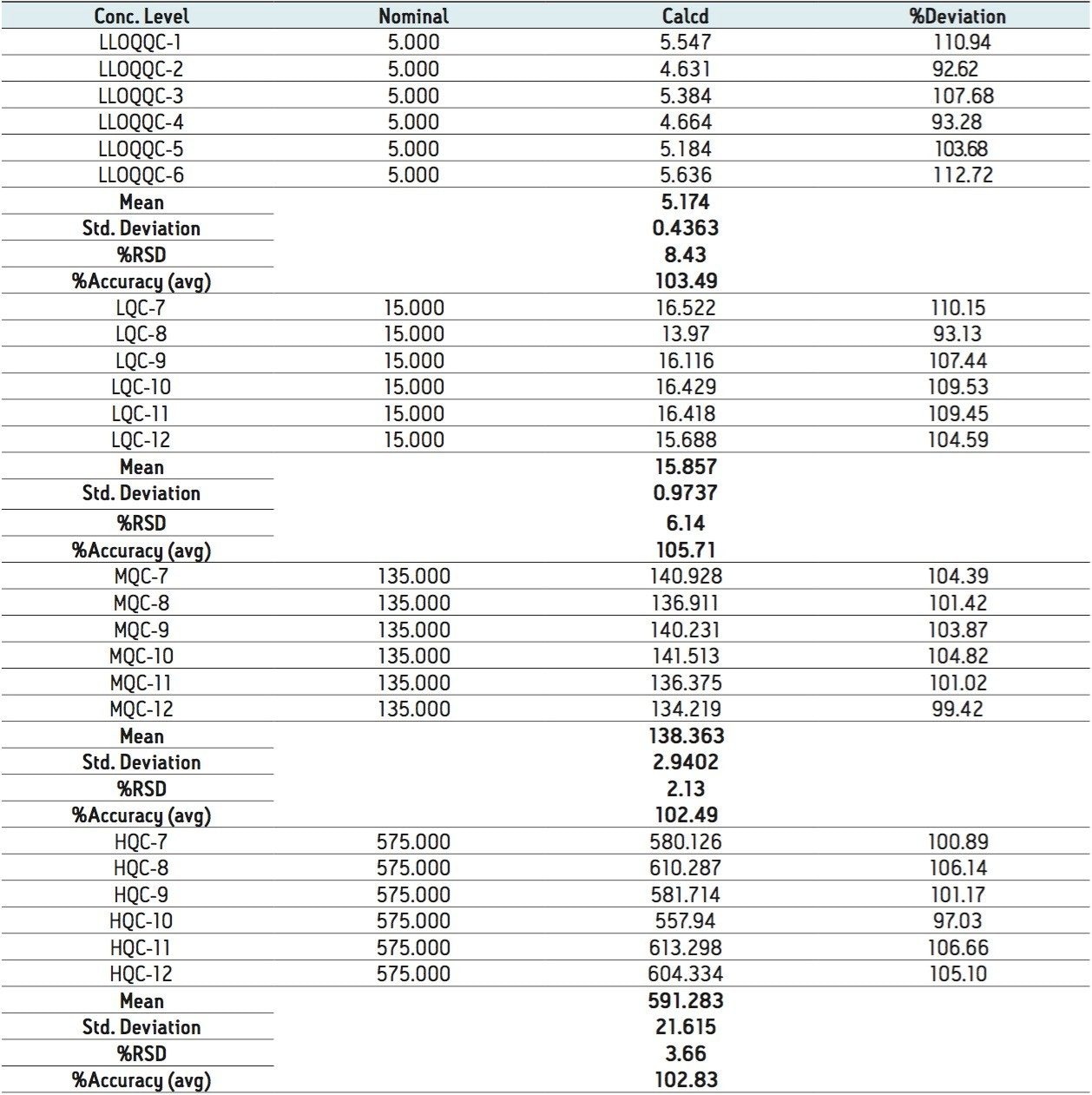
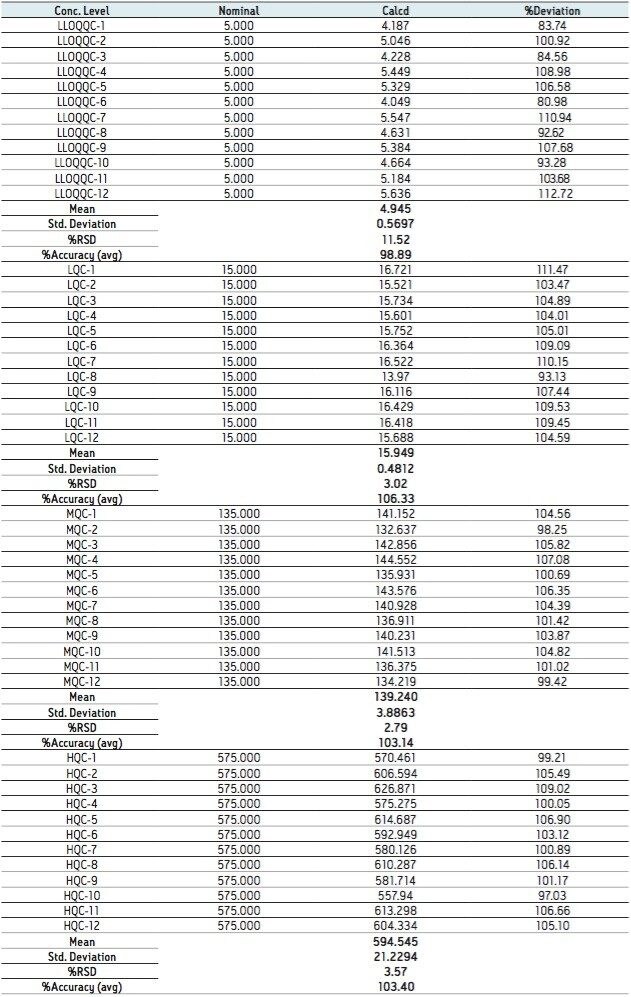
The data obtained for octreotide showed %RSD to the order of about 3% and did not vary significantly within three different concentration ranges (Figure 3 and Appendix, Table 1). In addition, the accuracy and %RSD observed at the LLOQ level was in good agreement with that observed for the three other concentration ranges (Figure 3 and Appendix, Table 1). The accuracy and precision at different concentration ranges of octreotide were in excellent agreement within two different batches of samples that were analyzed (Figure 4 and Appendix, Table 2).
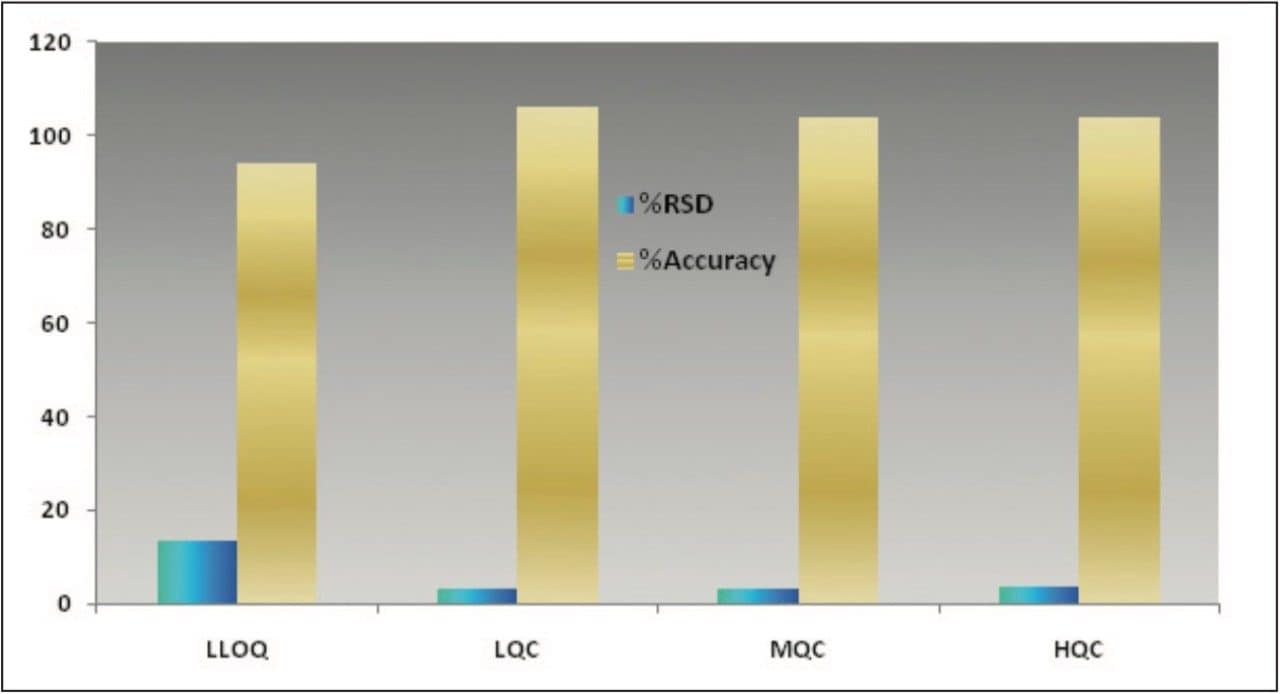
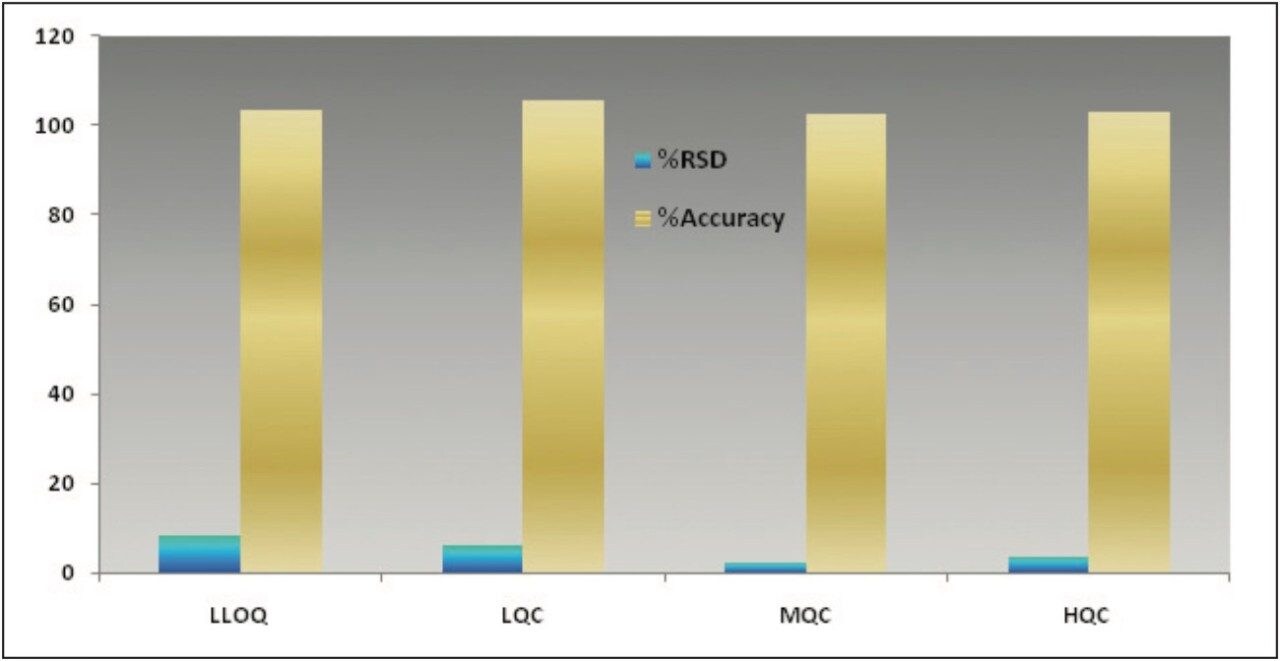
In addition, the global precision did not vary significantly between the three different concentration ranges and the accuracy of the results was within acceptable range (Figure 5 and Appendix, Table 3). Such high degree of precision and accuracy and low %RSD can be attributed to the robustness, reproducibility, and sensitivity of the combination of Waters technologies including best in class sample preparation capability, ACQUITY UPLC, and Xevo TQ-S.
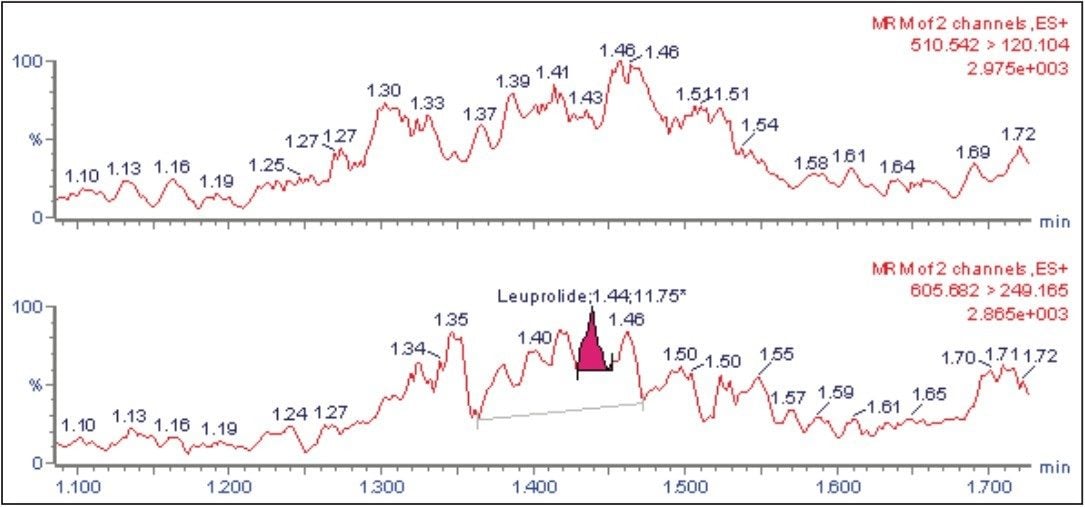
The UPLC-MS/MS chromatogram of the blank plasma sample and that of the octreotide at LLOQ level showed little interference from the endogenous materials (Figures 6A and Figure 6B). In addition, the phospholipid elution was checked by injecting an extracted standard with an MS scan at 184 ⇒ 184 m/z, a unique MRM transition for typical phospholipids (Figure 7). No significant phospholipid elution was observed at the retention time of either the octreotide analyte or its IS, leuprolide.
Such excellent separation of analyte of interest from any endogenous materials helps address some major regulatory issues, such as estimation of matrix interference. Once again, such abilities are achieved with an optimized combination of Waters sample preparation technology, chemistry, ACQUITY UPLC, and Xevo TQ-S.

The low circulating concentration levels of octreotide, a cyclic octapeptide that is used for treating cancer patients, requires a highly sensitive assay for accurate determination of the pharmacokinetics. However, such a highly sensitive analytical method with proper sample preparation protocols was not known for octreotide and hence its analysis in the low pg/mL concentration level was not previously achievable.
This study demonstrates that the combination of Waters Oasis SPE, an ACQUITY UPLC System with ACQUITY UPLC BEH 300 C18 Column, and the Xevo TQ-S Mass Spectrometer enables the development of an assay for octreotide with an LLOQ of 5 pg/mL in human plasma.
UPLC chromatograms not only demonstrate better sensitivity, but also better separation compared to any conventional HPLC instruments. The data in this study exhibit low %RSD, a high degree of accuracy, and excellent batch-to-batch reproducibility and, therefore, demonstrate the benefits of sensitivity, robustness, and reproducibility of this integrated bioanalytical system solution.
720004095, August 2011