This application note describes the use of Waters Xevo TQ MS for the specific detection of rbST in plasma samples collected from an animal treated with the recombinant hormone.
The results presented are amongst the very first allowing unambiguous detection of the administration of recombinant bovine somatotropin some days after injection.
Controlling safety with respect to food products of animal origin is a priority for governments, international regulatory bodies and organizations that process and handle products prior to consumption. Food safety issues arising from commodity products often become globally reported and have the potential to impact consumer confidence and trade at international levels.
Recombinant bovine somatotropin (rbST), also called growth hormone, is used in some countries as a general growth promoter in pigs and cattle but also in lactating cows to increase milk production1,2. This practice is strictly regulated, especially in the European Union, with a complete ban of this substance3,4. However, this regulation faces a lack of analytical tools able to detect the (mis)use of this hormone. The difficulty of analysis is due to the protein nature of the hormone, its low level of concentration in biological fluids, and the complexity of the matrices of interest. Up to now, methods have been limited to immunoassays with the problem that native and recombinant forms were not differentiated. A few attempts have been published but they were all unable to specifically detect rbST at physiological levels5,6.
Only recently, a successful strategy has been developed for the direct detection of rbST in biological matrices7,8. The analysis is based on the detection of the tryptic N-terminal peptide, specific of the recombinant form of the hormone. Indeed, the Alanine N-terminal amino acid present in the natural form is replaced by a methionine in the case of the recombinant hormone as present in formulations for injection.
This application note describes the use of Waters Xevo TQ MS for the specific detection of rbST in plasma samples collected from an animal treated with the recombinant hormone. The results presented are amongst the very first allowing unambiguous detection of the administration of recombinant bovine somatotropin some days after injection.
Protein standards of rbST and recombinant equine somatotropin reST were obtained from the Harbor-UCLA Medical Center, National Hormone and Pituitary Program (Torrance, USA) and Bresagen Limited (Thebarton, Australia), respectively.
Sample extraction and purification procedure for recombinant bovine somatotropin in plasma has already been described7,8.
|
LC system: |
ACQUITY UPLC System |
|
Runtime: |
8.00 min |
|
Column: |
ACQUITY BEH C18, 1.7 μm, 2.1 x 100 mm |
|
Porosity: |
130 A |
|
Mobile phase A: |
0.1% formic acid dissolved in water |
|
Mobile phase B: |
CH3CN + 0.1% formic acid |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
8.0 μL |
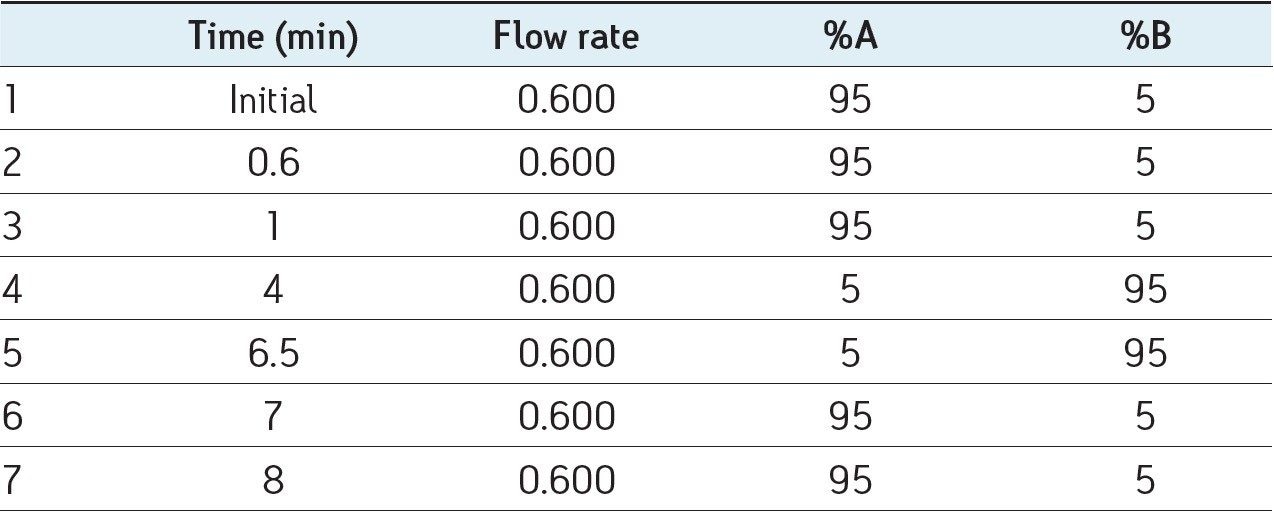
Mobile phase gradient is detailed in Table 1.
|
MS system: |
XEVO TQ MS |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3 kV |
|
Source temp: |
150 °C |
|
Desolvation temp: |
550 °C |
|
Desolvation gas: |
800 L/hr |
|
Collision gas flow: |
0.15 mL/min |
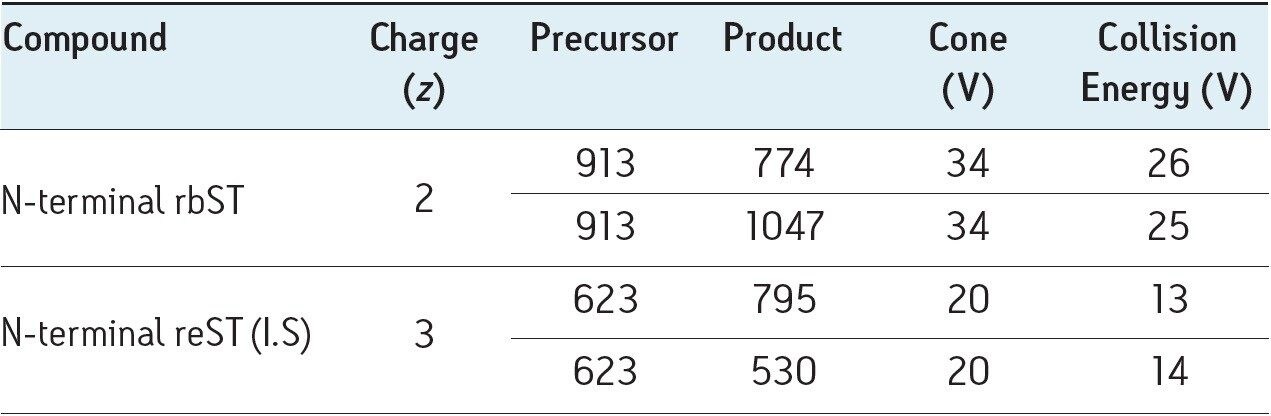
MRM transitions were first generated using Waters’ IntelliStart9. The various parameters were then optimized individually for each diagnostic signal. These can be found in Table 2.
The ACQUITY UPLC System allowed a good retention and separation of the tryptic N-terminal peptides with a total run time of 8 min, and retention times of 2.74 and 2.79 min for the N-terminal peptides rbST and reST, respectively (Figure 1).
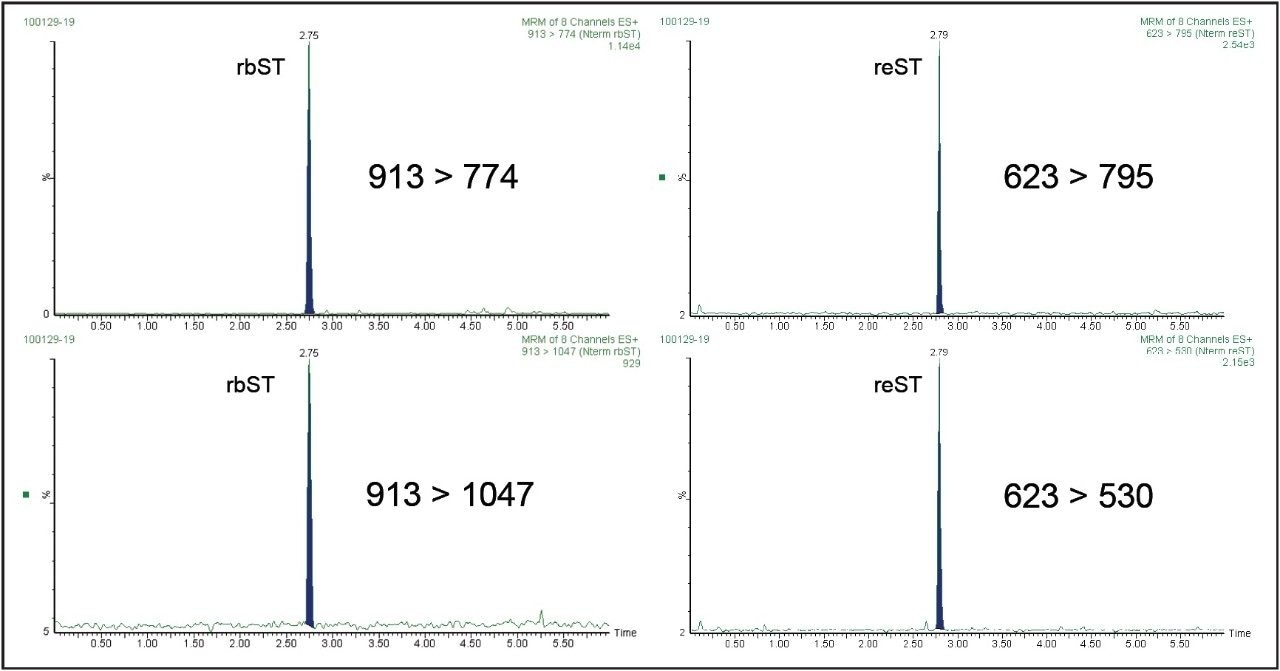
The positive electrospray ionization leads to a doubly-charged form for N-terminal peptide rbST: [M+2H]2+=913 which was selected as precursor ion. According to the Commission Decision 2002/657/EC10. Two diagnostic signals were selected in the MRM method for identification purposes. Recombinant equine somatotropin (reST) was used as internal standard with a fortification at 100 ng mL-1 in samples. The ionization of its N-terminal peptide lead to a main triply-charged form [M+3H]3+= 623, which was selected as precursor ion.
This method has been applied to samples collected from a treated animal. A lactating cow was treated once subcutaneously with a dose of 500 mg of Lactatropin.
The chromatograms corresponding to plasma samples collected before treatment(D0) and 2 days (D2) after treatment are shown in Figure 2.
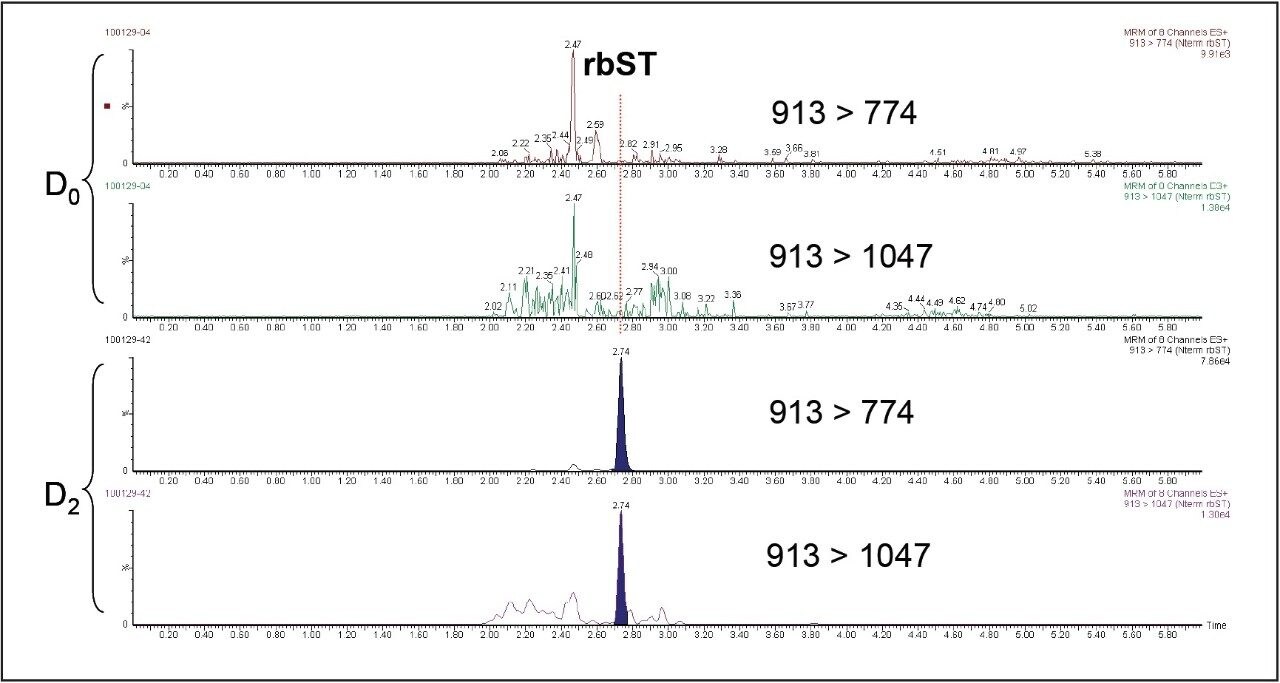
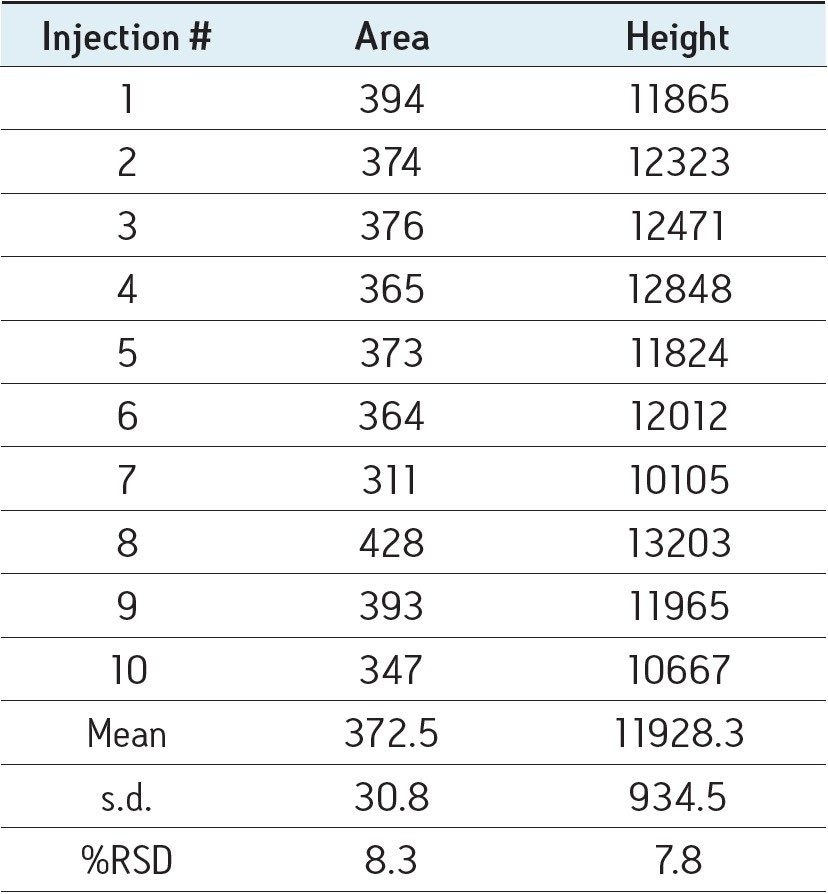
The results shown in Figure 2 show that it’s possible to detect rbST in plasma two days after its administration to the animal. Analysis of the signals acquired leads to unambiguous identification of the protein according to 2002/657/EC requirements10. This confirms the suitability of the detection method’s sensitivity in accordance with expected levels of the protein in animal plasma. Little evidence of matrix effects was observed and very good linearity in matrix allowed further relative quantification of rbST up to around 50 ppb in plasma two days after the administration of the hormone, which is in accordance with previous results7,11. The robustness of Xevo TQ MS allowed good repeatability at low levels in complex samples as shown in Table 3.
720003380, March 2010