This is an Application Brief and does not contain a detailed Experimental section.
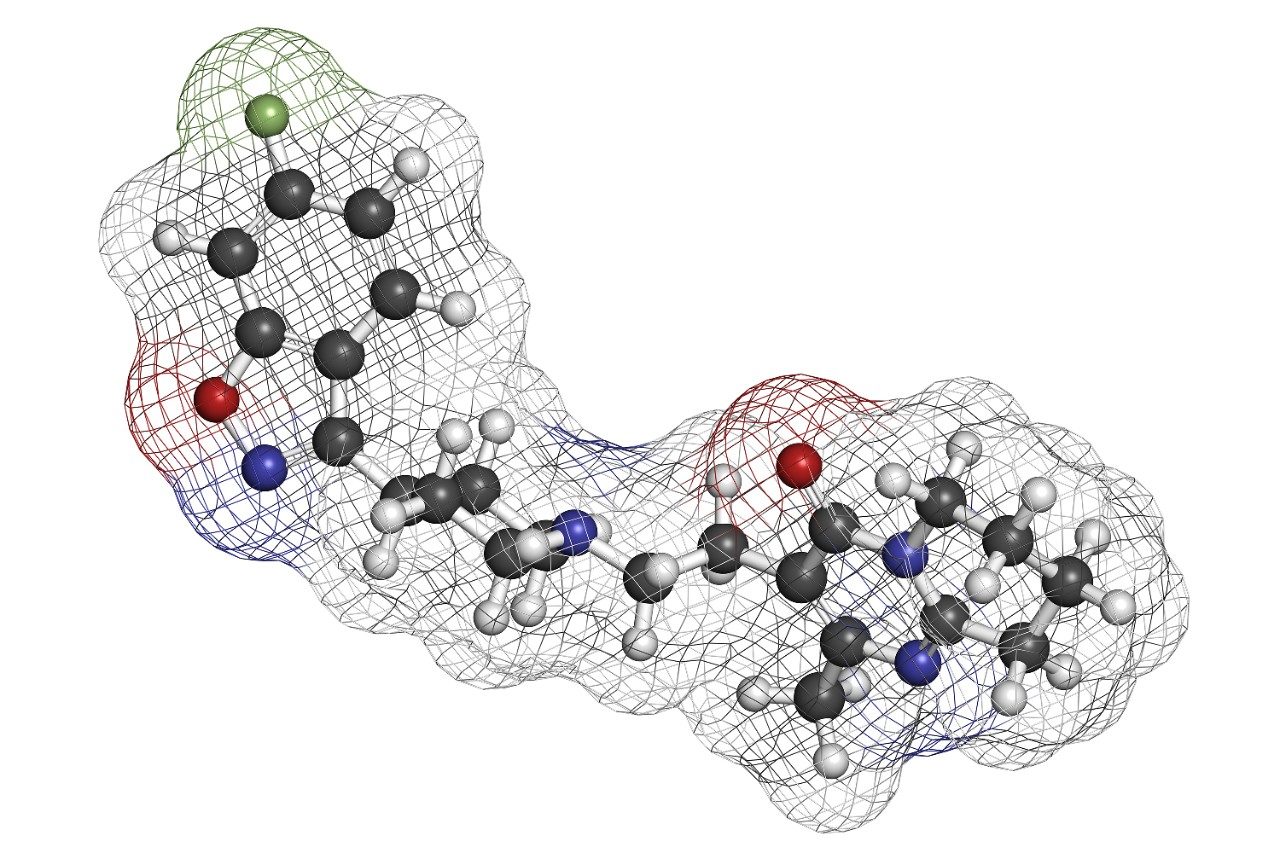
This application brief highlights the separation of risperidone and 9-hydroxyrisperidone on Oasis MCX.
Risperidone is an antipsychotic drug and is rapidly metabolized to the 9-hydroxyrisperidone metabolite in the liver. This metabolite is the predominant circulating species with same activity as the parent and therefore, must be quantitated. All analytes are bases and therefore an Oasis MCX plate was selected.

|
Oasis MCX 10-mg plate ( P/N 186000259) |
|
|---|---|
|
Condition: |
500 μL MeOH |
|
Equilibrate: |
500 μL H2O |
|
Load: |
500 μL (250 μL human plasma, diluted 1:1 with 4% H3PO4 in H2O) |
|
Wash 1: |
500 μL 2% FA |
|
Wash 2: |
500 μL MeOH |
|
Elute: |
250 μL (125 μL x 2) 5% NH4OH in MeOH |
|
Options: |
1. Dilute 250 μL H2O with 2% FA 2. Evaporate/ Reconstitute 3. Direct inject |
|
Inject: |
10 μL |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 50 mm, 1.7 μm |
|
Mobile Phase A: |
0.1% HCOOH in H2O |
|
Mobile Phase B: |
0.1% HCOOH in MeOH |
|
Flow Rate: |
0.3 mL /min |
|
Injection Volume: |
10.0 μ L |
|
Column Temperature: |
40 °C |
|
Sample Temperature: |
10 °C |
|
Instrument: |
ACQUITY UPLC with Quattro Premier |
|
Time (min) |
Profile |
|
|---|---|---|
|
%A |
%B |
|
|
0.0 |
60 |
40 |
|
1.0 |
60 |
40 |
|
1.5 |
0 |
100 |
|
3.5 |
0 |
100 |
|
4.0 |
60 |
40 |
|
4.5 |
60 |
40 |
|
Quattro Premier |
|
|---|---|
|
ESI+ |
|
|
Capillary: |
3.5 kV |
|
Source Temp.: |
120 °C |
|
Desolvation Temp.: |
350 °C |
|
Cone Gas Flow: |
0 L /Hr |
|
Desolvation Gas Flow: |
700 L /Hr |
|
Collision Cell Pressure: |
2.59 e-3 mbar |


WA60088, June 2007