This application highlights the development of a highly sensitive and robust LC-MS assay for the quantification of mometasone furoate extracted from plasma. The method described herein achieves an LLOQ of 0.5 pg/mL with a linear dynamic range of 0.5–60 pg/mL. This developed method has demonstrated its fit-for-purpose use in support of drug discovery and research.
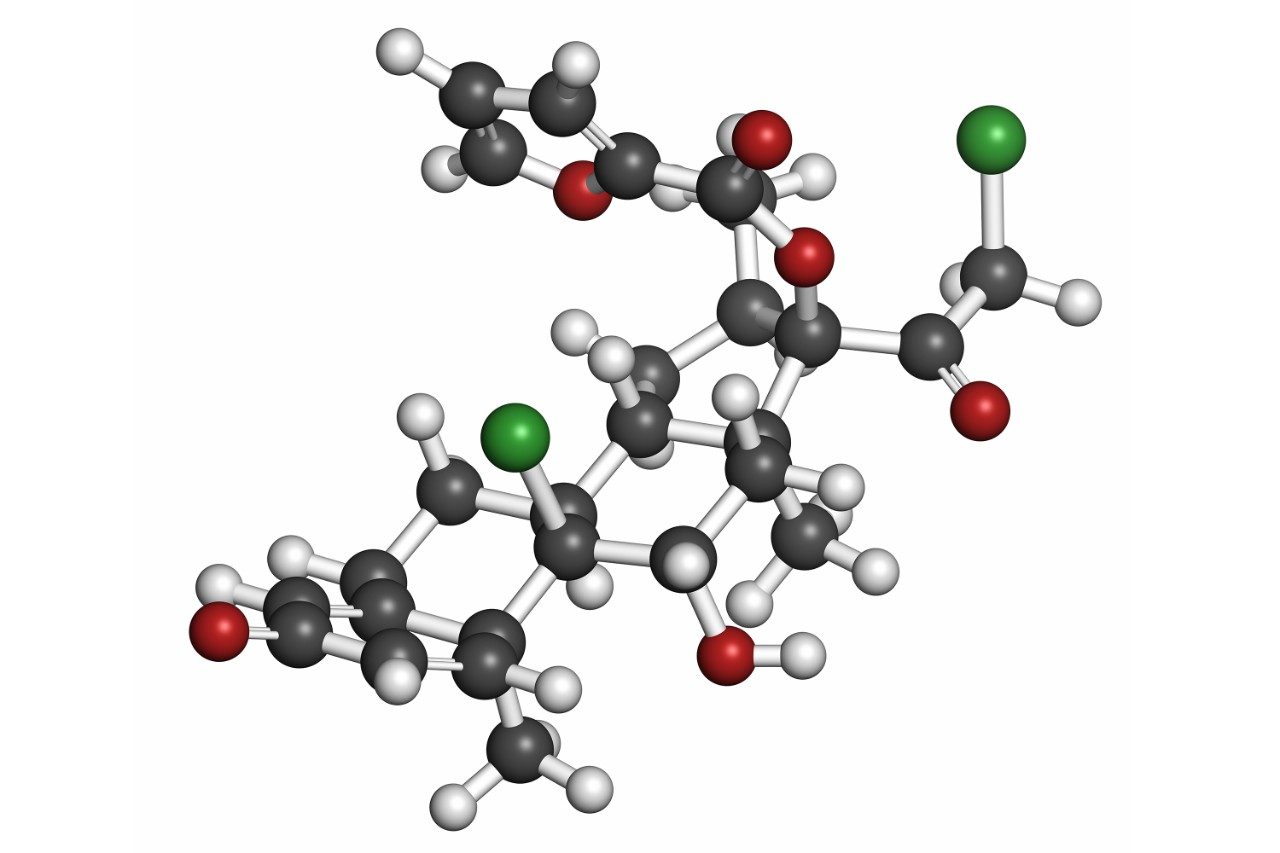
Mometasone furoate (Figure 1)1 is a synthetic corticosteroid with the chemical name 9, 21-dichloro-11(Beta),17-dihydroxy-16(alpha)-methylpregna1,4-diene-3,20-dione 17-(2-furoate). It is a potent beta 2-agonist with anti-inflammatory activity, used in the treatment of asthma and/or chronic obstructive pulmonary disease.2
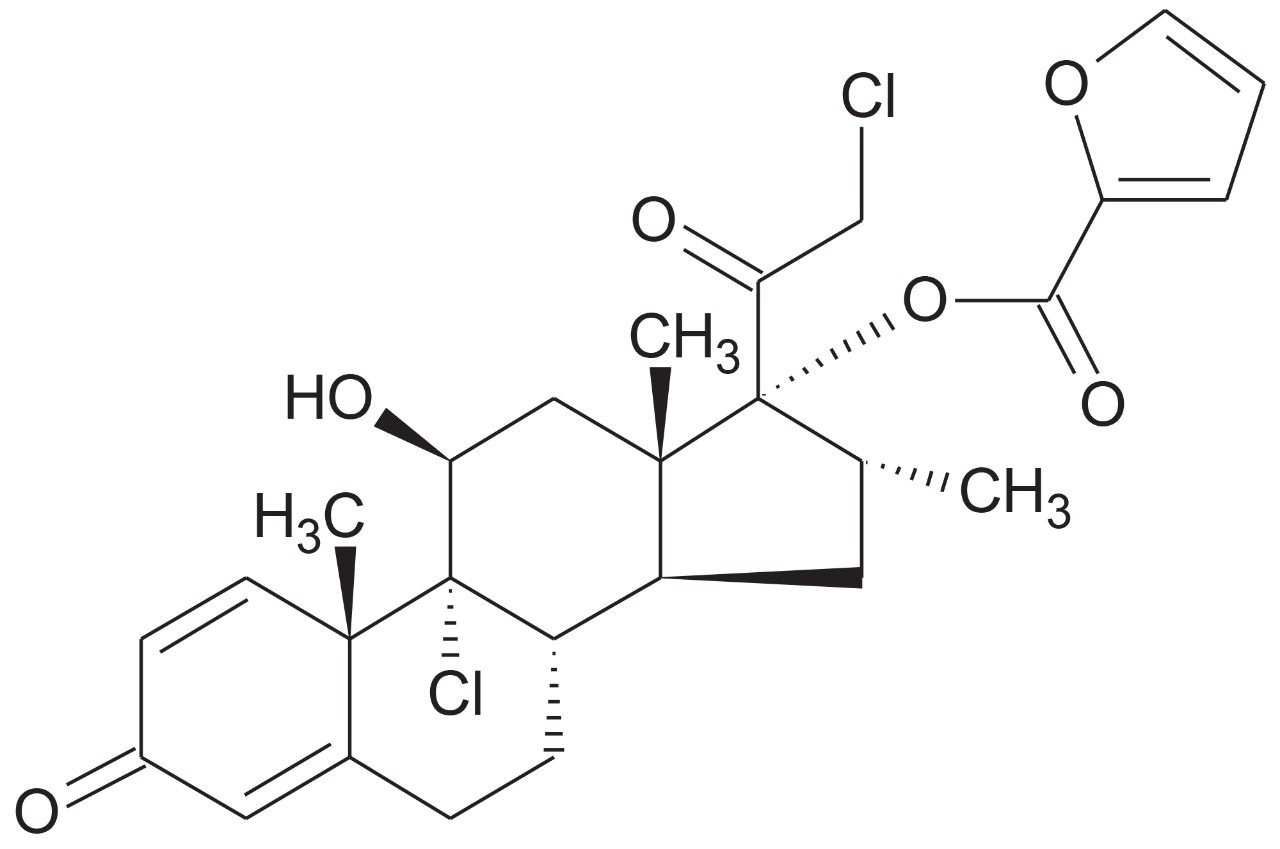
With minimal bioavailability (<1 %) and very low circulating plasma concentrations (50 pg/mL) following a 100–400 µg inhaled dose, accurate quantification of mometasone from plasma can be challenging.3 In this work, a robust, sensitive, and selective method was developed for the accurate quantification of mometasone furoate. This method uses UPLC separation, tandem quadrupole MS with UniSpray ionization, and selective solid-phase extraction (SPE) sample preparation, achieving a lower limit of quantitation (LLOQ) of 0.5 pg/mL from 600 µL of plasma.
Standards and quality control (QC) samples were prepared by spiking mometasone furoate (0.5 to 60 pg/mL) into commercially available human plasma. Calibration curve standards were prepared in duplicate to check the reproducibility, while five replicates were prepared for the QC’s. 25 µL of mometasone furoate-d3 (5 ng/mL), which was used as internal standard (ISTD), was added to the plasma samples spiked with mometasone furoate (600 µL). Following internal standard addition, sample was treated with 200 µL methanol and mixed. The pretreated plasma sample was then extracted using the Waters Oasis HLB 1 cc Cartridges and the extraction protocol shown in Figure 2. Following extraction, samples were injected for LC-MS/MS analysis.

|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY BEH Phenyl 130Å, 1.7 μm, 2.1 mm × 100 mm |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
0.1% Formic acid in 5 mm ammonium formate in water |
|
Mobile phase B: |
Methanol |
|
Reconstitution solution: |
50:50 Methanol:water |
|
Mass spectrometer: |
Xevo TQ-XS Tandem Quadrupole |
|
Ionization: |
ESI+ with UniSpray ion source |
|
Capillary voltage: |
0.5 KV (+) |
|
Desolvation temp.: |
650 °C |
|
Cone gas flow: |
150 L/h |
|
Desolvation gas flow: |
1000 L/h |
|
Collision cell pressure: |
3.8 × e-3 mbar |
|
Chromatography software: |
MassLynx |
|
Quantification software: |
TargetLynx |
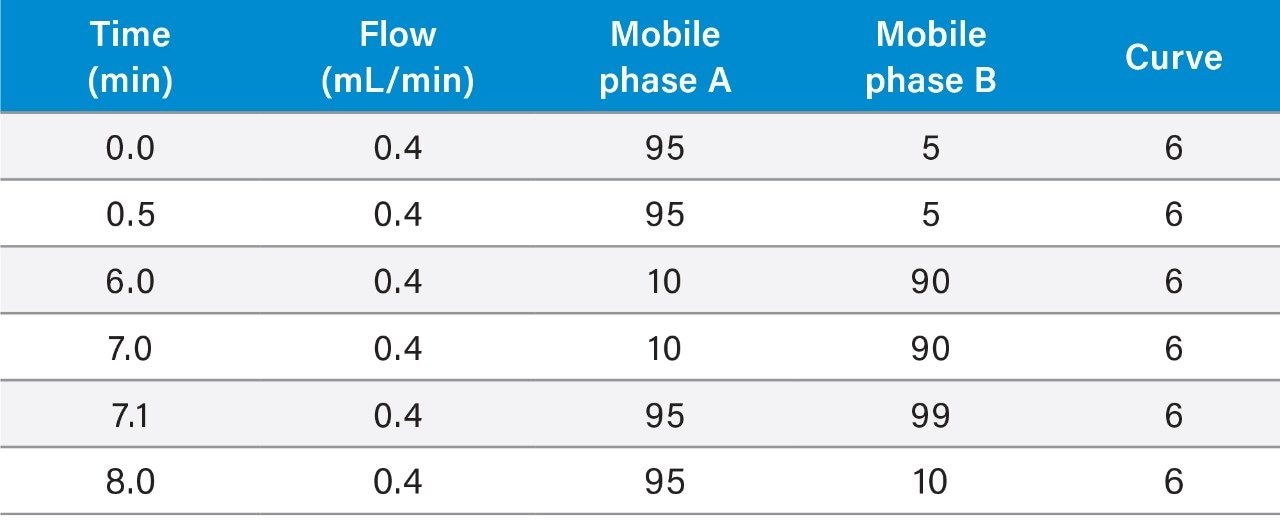
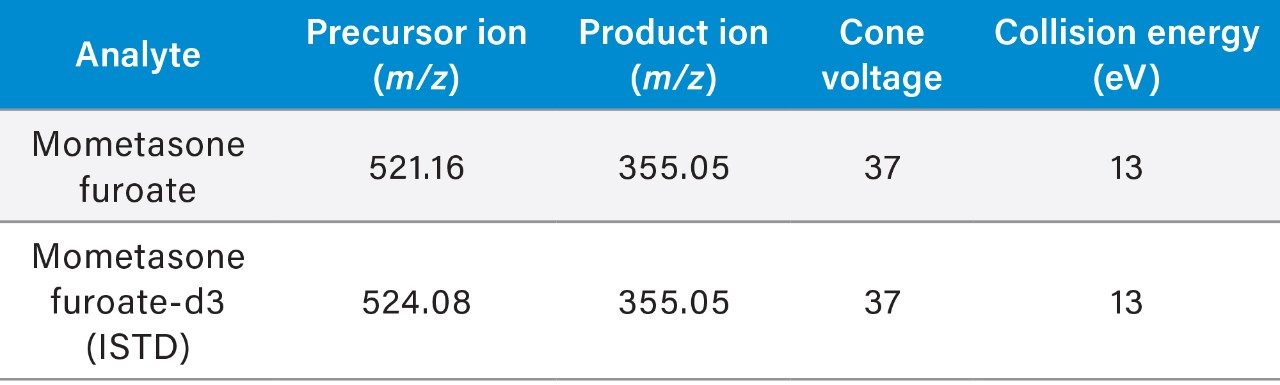
In this work, we have developed a complete sample preparation and UPLC LC-MS/MS method for sensitive and accurate quantification of mometasone furoate from plasma. SPE extraction of mometasone furoate from plasma was achieved using Oasis HLB 1 cc Cartridges using the SPE extraction procedure shown in Figure 2. Oasis HLB is a polymeric reversed-phased sorbent that provides high capacity and added specificity to this method. Recovery for mometasone furoate using this SPE extraction method was ~85%. LC-MS/MS quantification was performed using a Waters Xevo TQ-XS tandem/triple quadrupole mass spectrometer, coupled to an ACQUITY UPLC I-Class System. Chromatographic separation was achieved with an ACQUITY UPLC BEH Phenyl 1.7 µm Column, at a flow rate of 0.4 mL/min using a linear gradient (Table 1) with 5 mm ammonium formate in water containing 0.1% formic acid and methanol mobile phases. MRM transitions used for quantification are summarized in Table 2.
The Xevo TQ-XS Mass Spectrometer, equipped with a step-wave ion guide and UniSpray ion source, enabled improved ion sampling in the source, better ion transfer efficiency, and improved ionization. Use of a low dispersion UPLC I-Class System and sub-2-µm UPLC column, coupled to the Xevo TQ-XS, afforded excellent resolution from endogenous matrix components, enhancing selectivity, and sensitivity. This sensitivity and selectivity is illustrated in Figure 3 for the LLOQ, 0.5 pg/mL extracted plasma sample, achieving a signal-to-noise (S/N) ratio of 19.7.

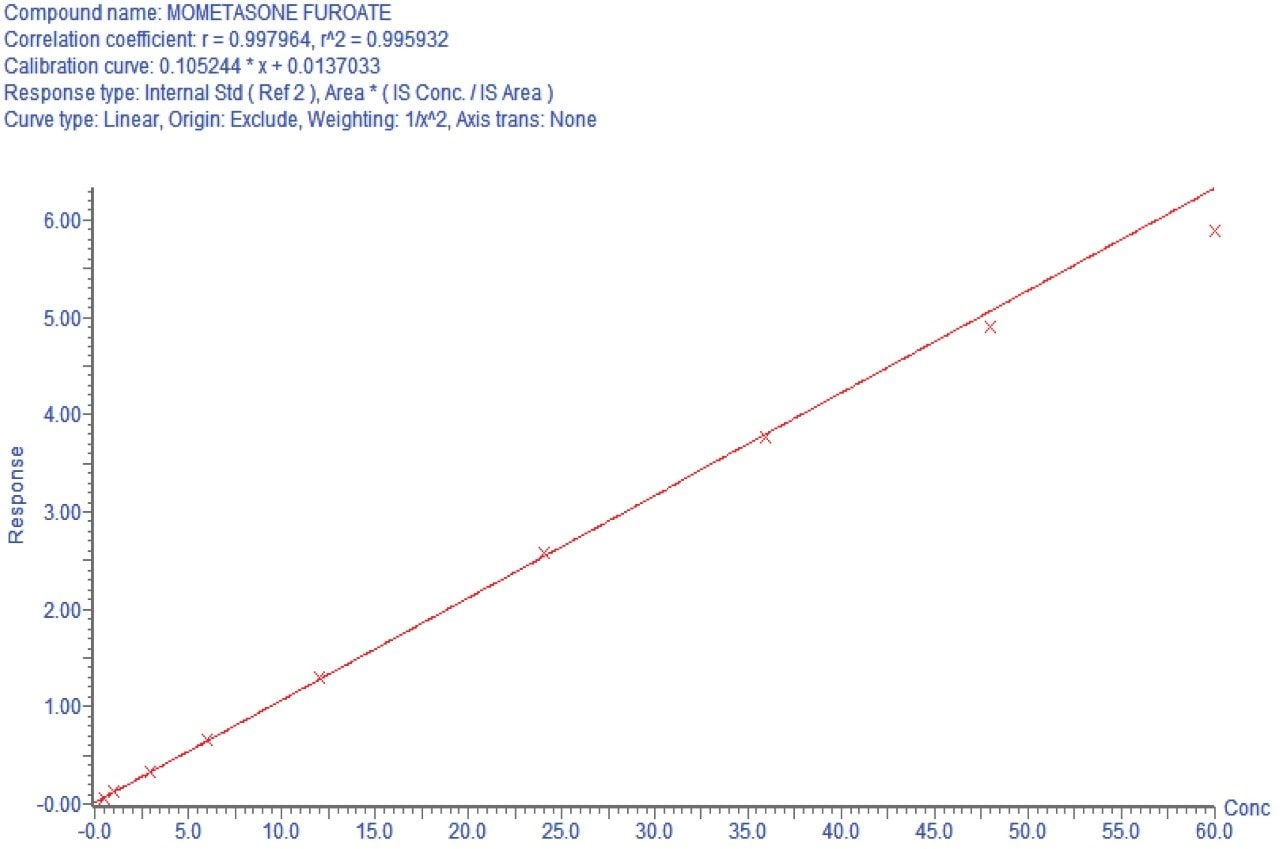
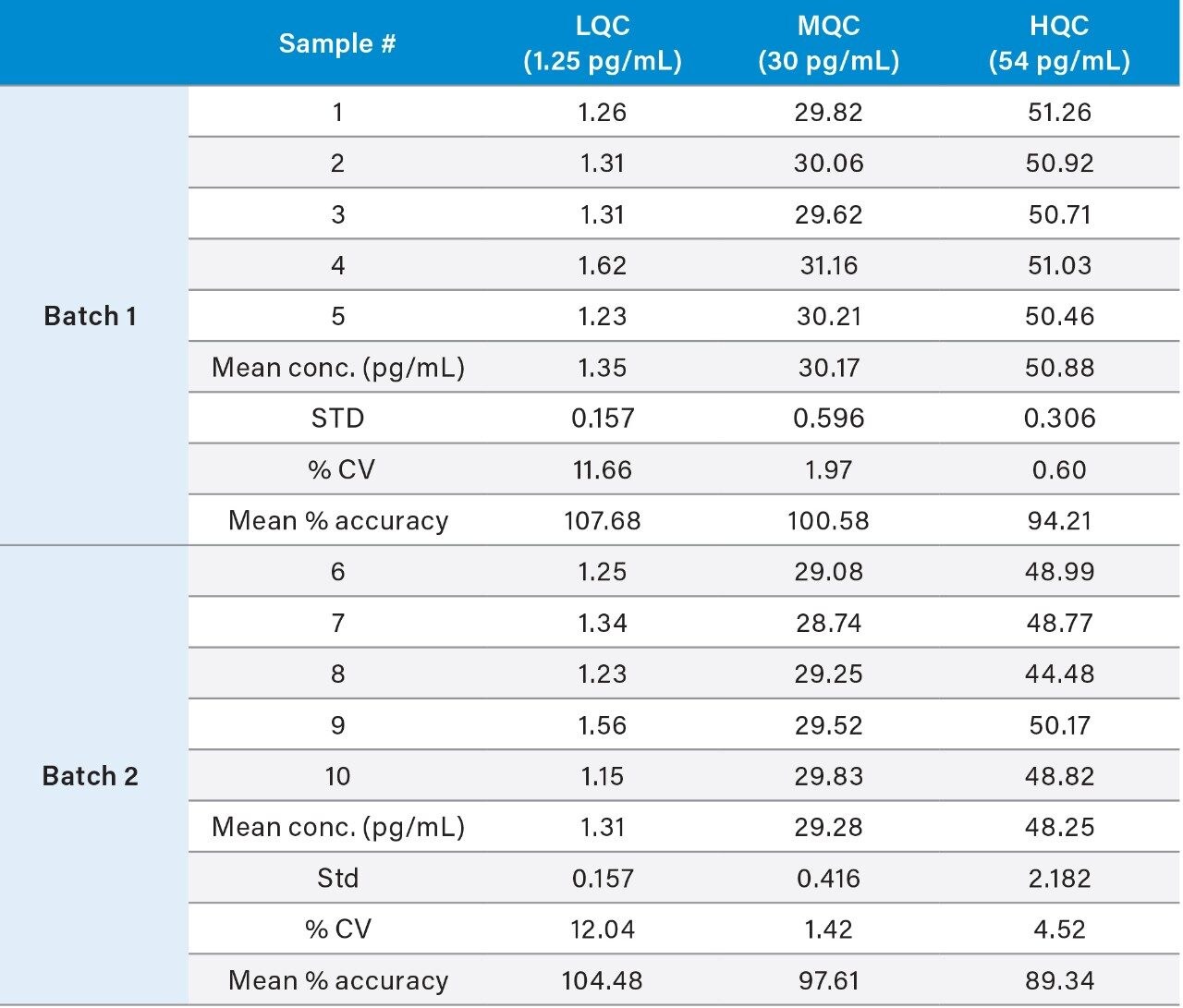
Using this SPE LC-MS method, quantitative performance was excellent. Calibration curves were linear (r2>0.9959) from 0.5–60 pg/mL with accuracies between 85–115% and CVs <15% for all points on the curve. Figure 4 illustrates this performance. At the same time, QC statistics easily met recommended bioanalytical method development guidelines,4 with average precision and accuracy values <15%. This QC performance is highlighted in Table 3 for precision and accuracy (PA) batches, while chromatographic performance is illustrated in Figure 5.

This application highlights the development of a highly sensitive and robust LC-MS assay for the quantification of mometasone furoate extracted from plasma. The method described herein achieves an LLOQ of 0.5 pg/mL with a linear dynamic range of 0.5–60 pg/mL. The high sensitivity and linearity of method was attributed to extraction specificity of Oasis MCX solid phase extraction sample preparation, high resolution UPLC chromatographic separation with the ACQUITY UPLC I-Class System using a sub-2-µm UPLC column, and sensitivity of the Xevo TQ-XS Mass Spectrometer with UniSpray Technology. This developed method has demonstrated its fit-for-purpose use in support of drug discovery and research.
720006399, September 2018