Trace impurities in synthetic products that interact with human end users or may have an undesirable environmental fate are regulated by various government agencies such as FDA and EPA. As a result, impurity separation and their structural identification are important research functions for many industries, including pharmaceutical, agrochemical, food, and consumer products. Full chemical identification requires structural elucidation of the separated compound using high resolution mass spectrometry (HRMS). However, MS alone is often insufficient to unambiguously identify a compound, especially in the case of isomers. This often necessitates obtaining the isolated pure compounds of interest using purification procedures for study using NMR spectroscopy. In this application note, we describe how a workflow to achieve the full structural elucidation of trace impurities can be implemented using preparative supercritical fluid chromatography to isolate trace impurities.
Trace impurities in synthetic products that interact with human end users or may have an undesirable environmental fate are regulated by various government agencies such as FDA and EPA. As a result, impurity separation and their structural identification are important research functions for many industries, including pharmaceutical, agrochemical, food, and consumer products. Full chemical identification requires structural elucidation of the separated compound using high resolution mass spectrometry (HRMS). However, MS alone is often insufficient to unambiguously identify a compound, especially in the case of isomers. This often necessitates obtaining the isolated pure compounds of interestusing purification procedures for study using NMR spectroscopy.
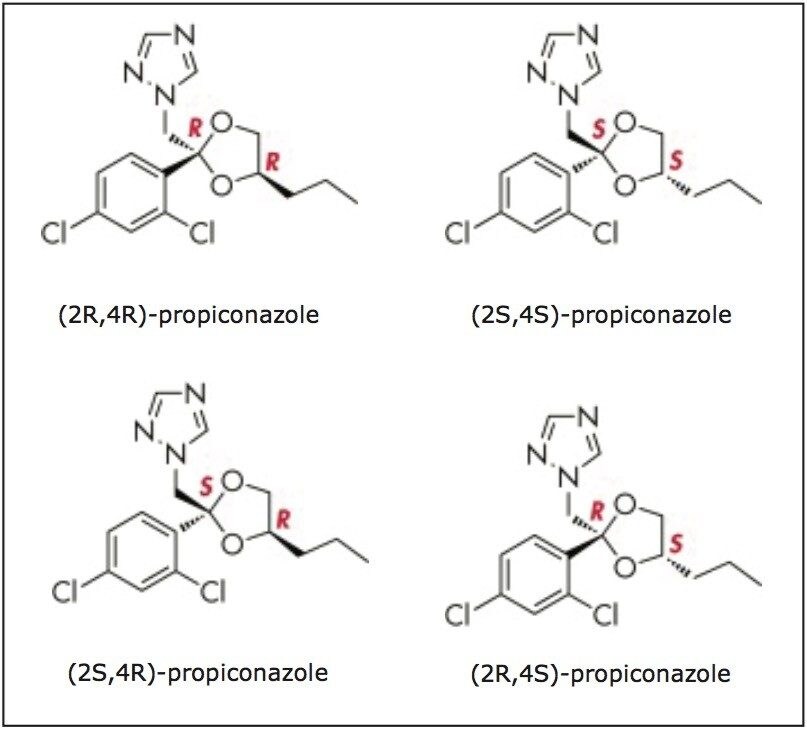
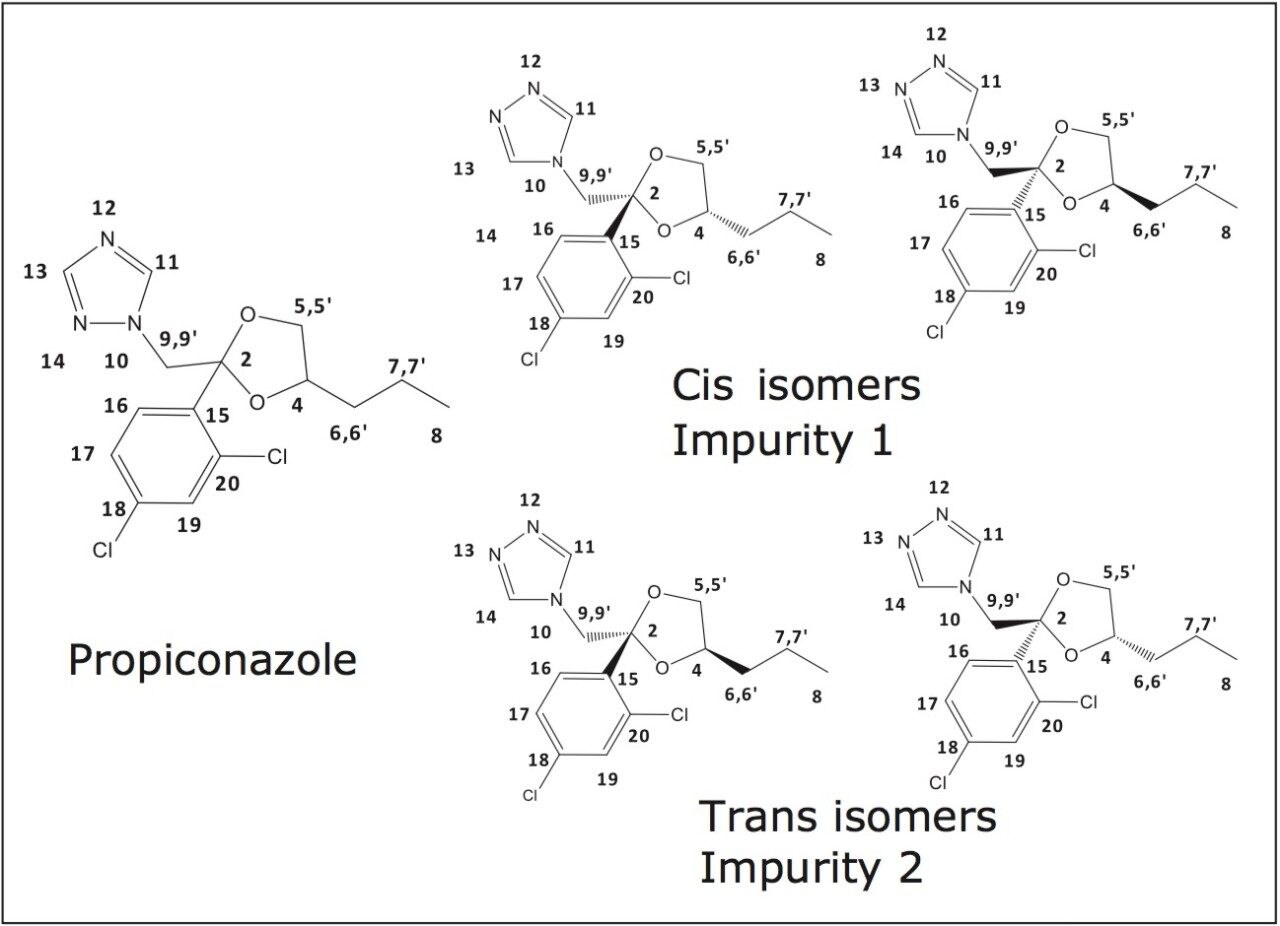
In this application note, we describe how a workflow to achieve the full structural elucidation of trace impurities can be implemented using preparative supercritical fluid chromatography to isolate trace impurities. A commercial formulationof the fungicide propiconazole was used as an example to demonstrate this workflow.1 Propiconazole has the structural potential for the existence of several stereoisomers. The propiconazole product used contains isomers (Figure 1) and also related trace impurities at approximately the 1% level (Figure 2). Some propiconazole impurities have previously been structurally identified.2 The SFC based workflow described here is generally applicable for impurity isolation and offers many advantages, including high speed and efficiency, fast dry-down, quick turnaround time, as well as the environmentally sustainable benefit of lower solvent consumption.3
A representative sample was obtained from a commercial formulation containing 1.55% of propiconazole and its trace impurities, using liquid-liquid extraction with dichloromethane (DCM) and a 5% sodium bicarbonate solution (NaHCO3).
(See Table 1)
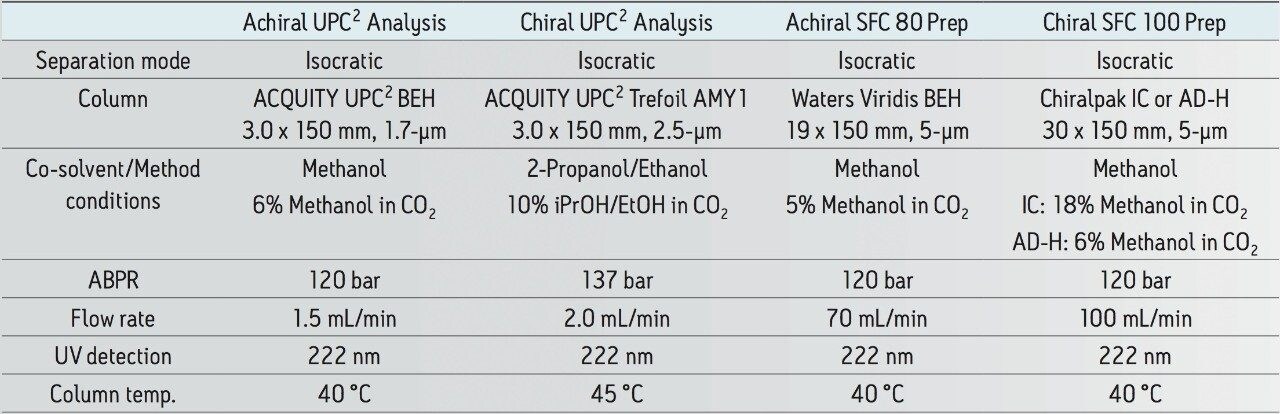
Mass spectral detection was performed on a Waters SQ Detector 2 in ESI mode scanning from 200 to 700 Da with a capillary voltage of 3 kV, cone voltage of 30 V, source temp. of 150 °C, and a desolvation temp. of 450 °C using an extracted ion at 342 Da, (M+H)+.
1H 13C, and 2D NMR spectra were acquired at 400 MHz using a Bruker Avance III spectrometer at the COSMIC Laboratory at Old Dominion University in Norfolk, VA. Assignments were based on chemical shifts, splitting patterns, integration of the peaks, coupling constants, coupling patterns in a 2D COSY spectrum (Correlated Spectroscopy) and one-bond carbon-proton coupling correlations observed in an HSQC spectrum (Heteronuclear Single Quantum Coherence) edited for carbon multiplicities. In a 2D NOESY spectrum (Nuclear Overhauser Effect Spectroscopy), NOE (Nuclear Overhauser Effect) was observed between the protons at carbons 11 and 14 of the triazole and the protons on carbon 6. The same NOE signal was observed in a 1D NOE spectrum with selective irradiation of the triazole protons in impurity peak 1 from the ACQUITY UPC2 BEH Column. There were no equivalent NOE signals observed in impurity peak 2.
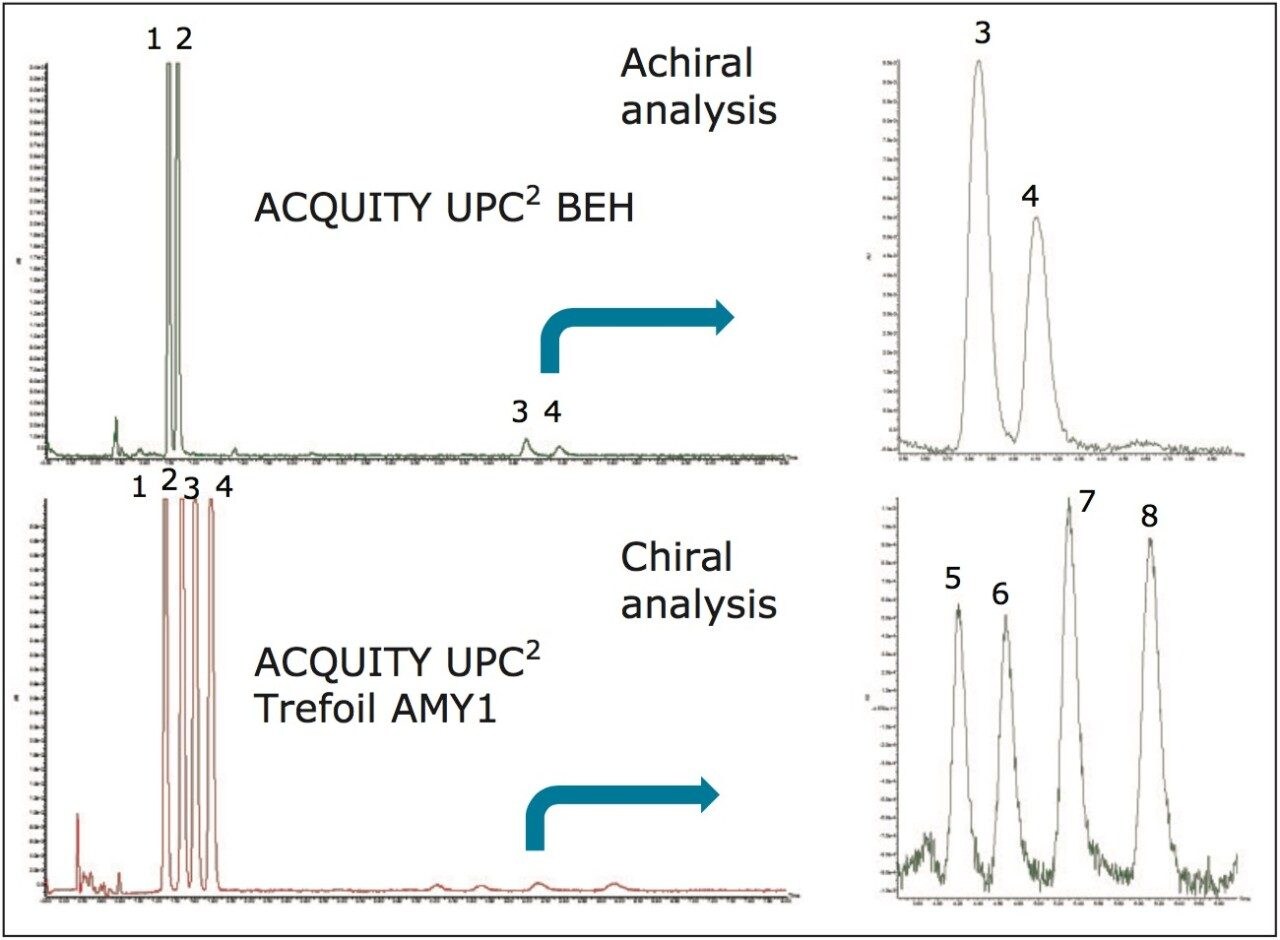
A commercial fungicide formulation (150 mL) containing propiconazole as the active ingredient at a concentration of 1.55% was suspended in 5% NaHCO3 solution then extracted with DCM three times. The combined extracts were dried over sodium sulphate (Na2SO4), filtered, then concentrated. The expected amount (2.3 g) of active ingredient (AI) was contained in 4.5 g of crude extract that contained other inactive ingredients including various surfactants. These crude extracts were then analyzed using UPC2. In both achiral and chiral modes (Figure 2) trace impurities at <1% of the amount of the original 1.55% claim were observed.
Methods were then developed to scale separations to preparative chromatography. The achiral prep chromatography was carried out using an SFC 80q Preparative System with a 19 mm I.D. Viridis BEH Column. The analysis showed that the two impurity peaks were obtained in 98% purity (Figure 3).
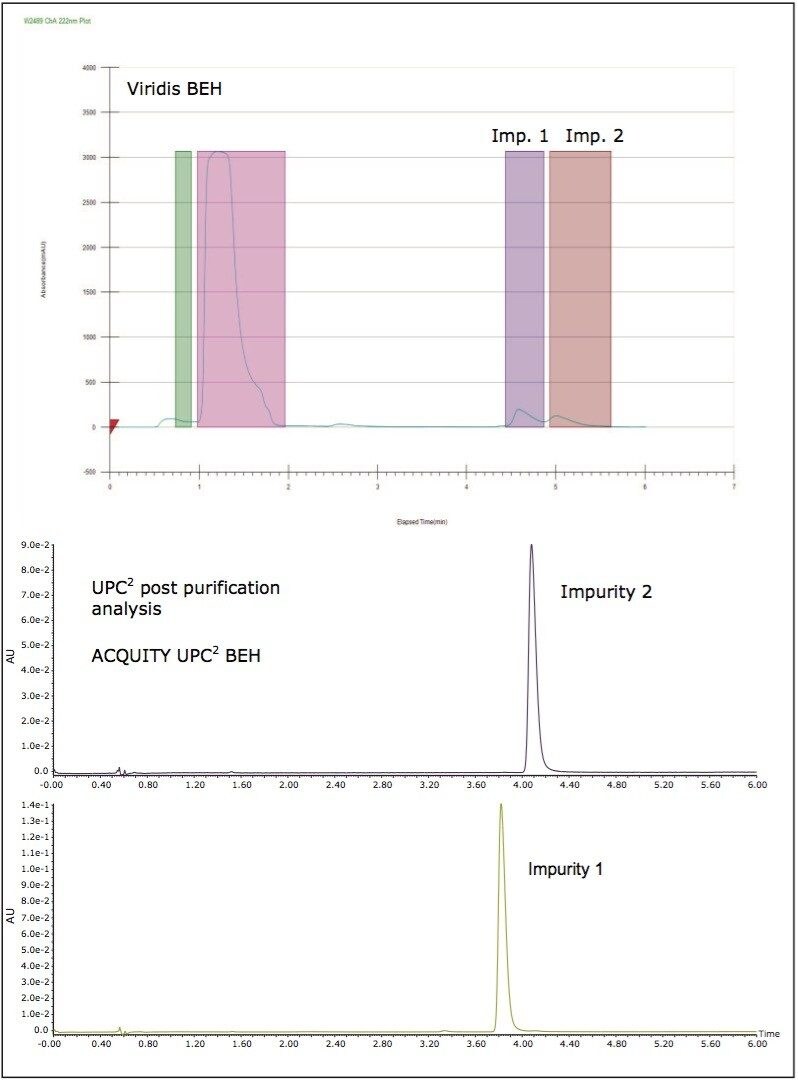
Sufficient pure material was collected both to enable full structural assignments of the trace impurities as well as to carry out further chiral separations of each peak into their individual enantiomers. MS analysis confirmed that the two isolated impurity peaks were isobaric with each other as well as with the main ingredient suggesting that they were indeed structural isomers.
1H, 13C, 2D, and NOE NMR experiments revealed that these two impurities differed from propiconazole itself by the nitrogen attachment point of the triazole moiety to the methylene group on the dioxolane ring (Figure 4). This result is clearly evident due to symmetry in the NMR. The propiconazole, being attached at the N adjacent to the other N is not symmetric, while in the impurity the N has a C atom adjacent on both sides of the nitrogen, giving symmetry to the triazole moiety and a simpler NMR. The assignment of the cis and trans isomers results from the fact that a strong NOE is seen in both a 2D NOESY NMR experiment as well as in a 1D NOE spectrum between the protons attached to carbons 11 and 14 and those on carbon 6 in the isomer assigned as cis, while this same NOE was not observed in the NMR spectrum of the isomer assigned as trans.
Having completed the full structural assignments for the two impurity peaks that could be resolved with achiral chromatography, attention was next turned to preparatively separating the enantiomers for both the cis and trans isomers (Figure 5). While all four isomers can be resolved in a single run analytically on a small particle size chiral column, in this case two different column chemistries were required for the separation of each enantiomeric pair upon scale up. The cis isomer separated on the IC column chemistry while the trans isomer required an AD-H column. All four of the possible impurity stereoisomers were then obtained in their pure form.
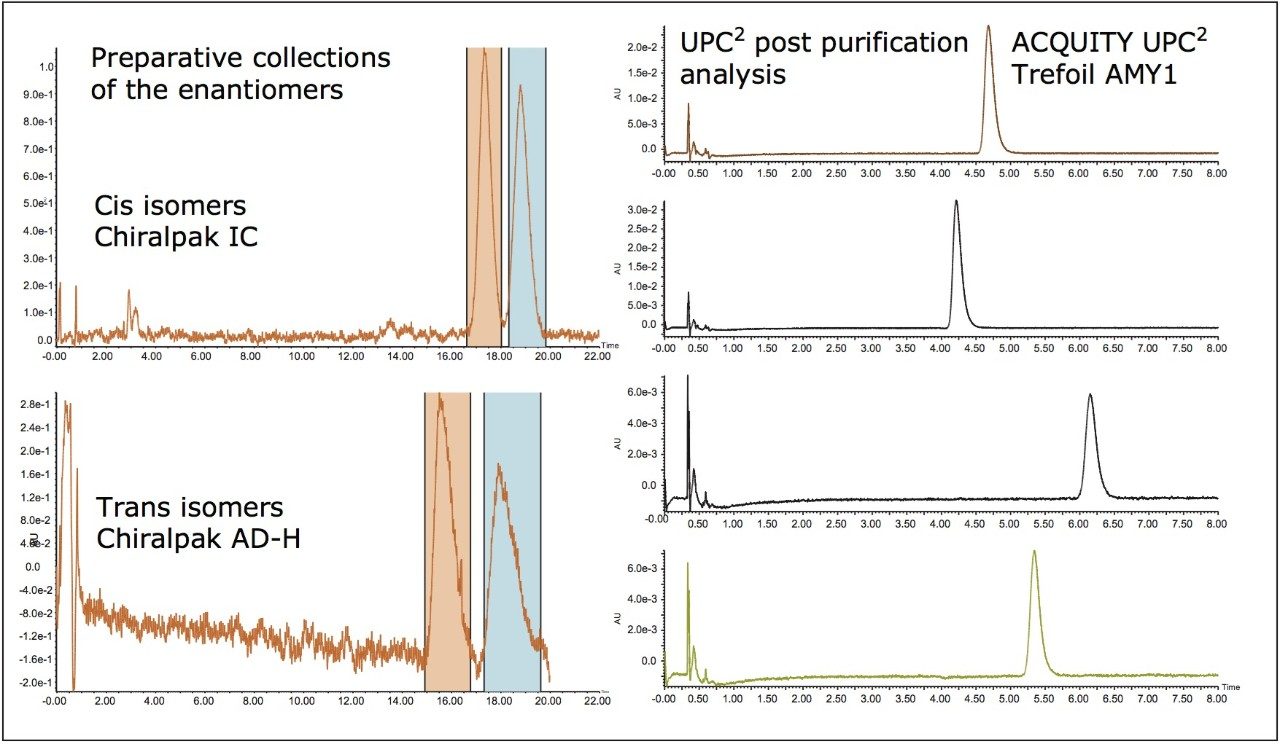
Chiral analysis using UPC2 demonstrated that each isomer had an enantiomeric excess >98%. The cis isomer was comprised of the 2R, 4S and the 2S, 4R isomers, while the trans isomers contained the 2R, 4R and the 2S, 4S isomers. Although the assignment of the absolute configurations for each of the stereoisomers was beyond the scope of this study, these isolated impurities do in fact possess the same structures as those found in a previous study.2 The capability to have all four of the impurity stereoisomers in hand in their pure form, in sufficient supply, then allows for the unambiguous assignment of the mechanistic potencies and toxicities of each individual impurity species. This capability then enables improved knowledge of the efficacy and safety of the product mixture ingredients.
ACQUITY UPC2 analyses of actual product mixtures, using both chiral and achiral column chemistries are useful for both impurity profiling and also for developing separation methods suitable for eventual scale up to preparative separations.
Trace impurities, as in this case <1% of a 1.55% formulation of the active ingredient, can be efficiently isolated in the amounts needed for structural elucidation and other needed studies using preparative SFC.
The isolated impurities can be obtained in their pristine forms, thus enabling the simple application of HRMS, 1D, and 2D NMR spectroscopic studies, allowing for the full structural elucidations of these trace impurities.
Initially isolated impurities can be further separated into their enantiomeric pairs through the implementation of chiral stationary phase preparative SFC with high efficiency.
The assigned structures for all of the isolated impurities were in agreement with information in the prior literature.2
Special thanks for the intellectual contributions of James E. Hall, NMR Spectroscopist, Old Dominion University, Norfolk, VA.
720005347, April 2015