
For research use only. Not for use in diagnostic procedures.
This application note details the use of mixed-mode ion exchange solid phase extraction (SPE) to selectively isolate serotonin from urine, followed by chromatographic separation on a high-strength silica (HSS) PFP column.
Clinical research scientists often measure concentrations of urinary serotonin (5-HT). While serotonin is often analyzed by HPLC using electrochemical detection (ECD), the use of UPLC-MS/MS affords greater analytical selectivity. This application note details the use of mixed-mode ion exchange solid phase extraction (SPE) to selectively isolate serotonin from urine, followed by chromatographic separation on a high-strength silica (HSS) PFP column. The method, which utilizes orthogonal sample pretreatment and chromatography, minimizes matrix effects as it promotes resolution of serotonin from potentially interfering endogenous compounds.
A stock, standard solution of 1.0 mg/mL serotonin was prepared in methanol containing 0.1% formic acid (to discourage oxidation). A stock solution of internal standard composed of 500-μg/mL D4-serotonin (CDN Isotopes, Pointe-Claire, Quebec) was prepared in 50:50 methanol/water containing 0.1% formic acid. Working standard solutions were prepared daily in 10% methanol with 0.1% formic acid.
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC HSS PFP Column, 1.7 μm, 2.1 x 100 mm |
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Mobile phase A (MPA): |
Water with 0.1% formic acid |
|
Mobile phase B (MPB): |
Acetonitrile with 0.1% formic acid |
|
Purge solution: |
20% Acetonitrile |
|
Wash solution: |
5% Acetonitrile |
|
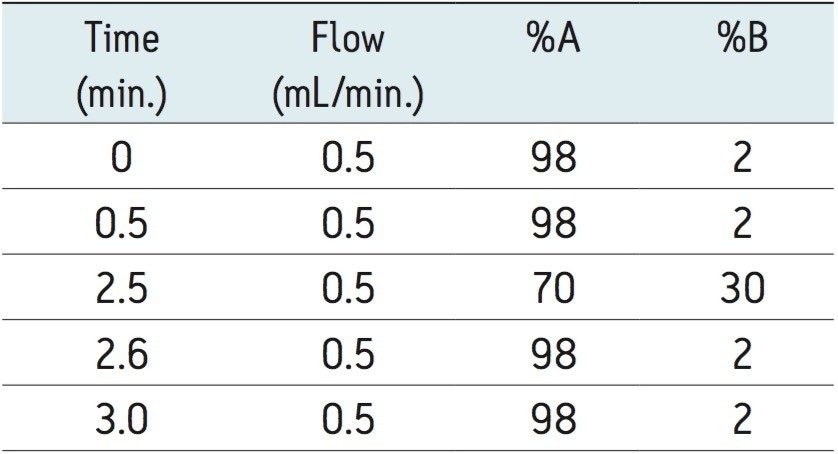
The gradient program is shown in Table 1. |
|
MS system: |
Xevo TQD |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
1.0 kV |
|
Cone voltage: |
22 V |
|
Desolvation gas: |
900 L/hr |
|
Cone gas: |
0 L/hr |
|
Desolvation temp.: |
500 °C |
|
Source temp.: |
150 °C |
Data were acquired and analyzed using MassLynx Software (V4.1, SCN 855). Quantification was performed using the TargetLynx Application Manager.
Urine samples were pretreated with 1.67% (v/v) 6 N HCl, to mimic the acidic pretreatment normally used for this method. 40 μL of a 1 μg/mL internal-standard working solution was added to a 400-μL aliquot of acidified urine, followed by 1 mL of 0.5 M NH4CH3COO. Pretreated samples were loaded in individual wells of a WCX 96-well plate that had been conditioned with 1 mL of methanol and 1 mL of water. After loading the samples, wells were washed with 1 mL of 20 mM NH4CH3COO followed by 1 mL of methanol. The 96-well plate was then dried under vacuum for 30 seconds, to remove as much methanol as possible from the sorbent bed. The samples were eluted from the plate with 2 x 250 μL aliquots of 30:70 methanol/water containing 5% formic acid into an 800-μL, 96-well sample collection plate (p/n 186002481). Each aliquot was initially allowed to percolate through the well, to maximize duration of contact with the sorbent. 5 μL of the eluate was injected onto the UPLC-MS/MS system. The extraction procedure is summarized in Figure 1.
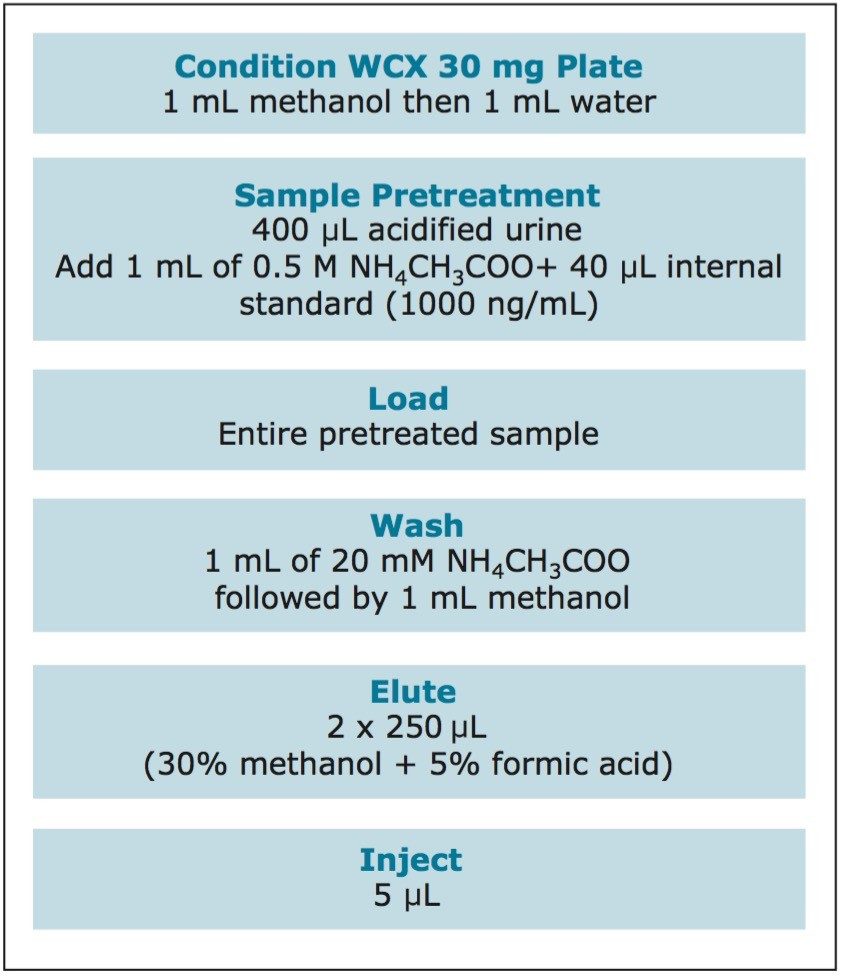
Table 2 shows the retention time and individualized MS parameters of serotonin and its deuterated internal standard, including MRM transitions, cone voltage, and collision energy.
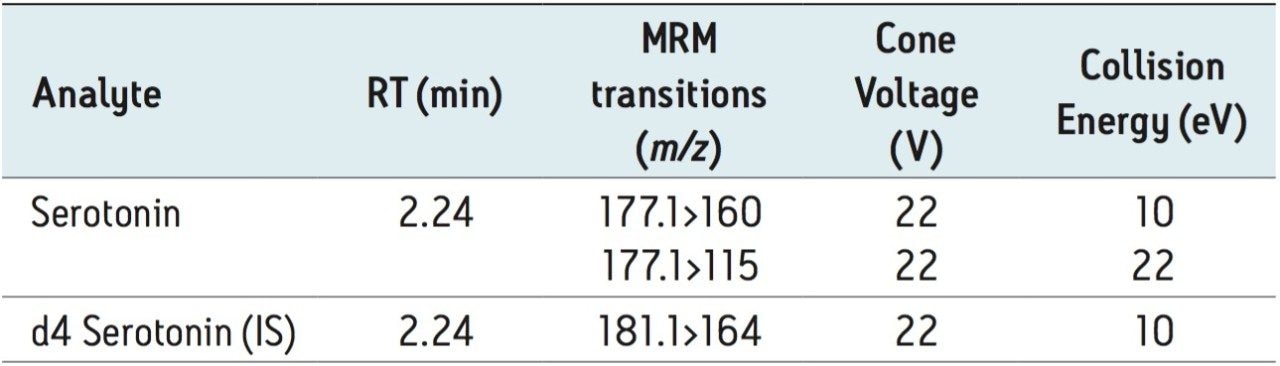
Figure 2 shows the chromatography of serotonin and its internal standard from an extracted method blank (A) and a 50 ng/mL calibration standard (B) using the ACQUITY UPLC HSS PFP Column. The serotonin peak seen in the method blank represents the endogenous concentration of serotonin (29.7 ng/mL) contained in the pooled urine used to create the calibration curve. The PFP column chemistry provided excellent retention and peak shape as well as baseline resolution from the endogenous, potentially interfering peaks that elute close to that of serotonin.
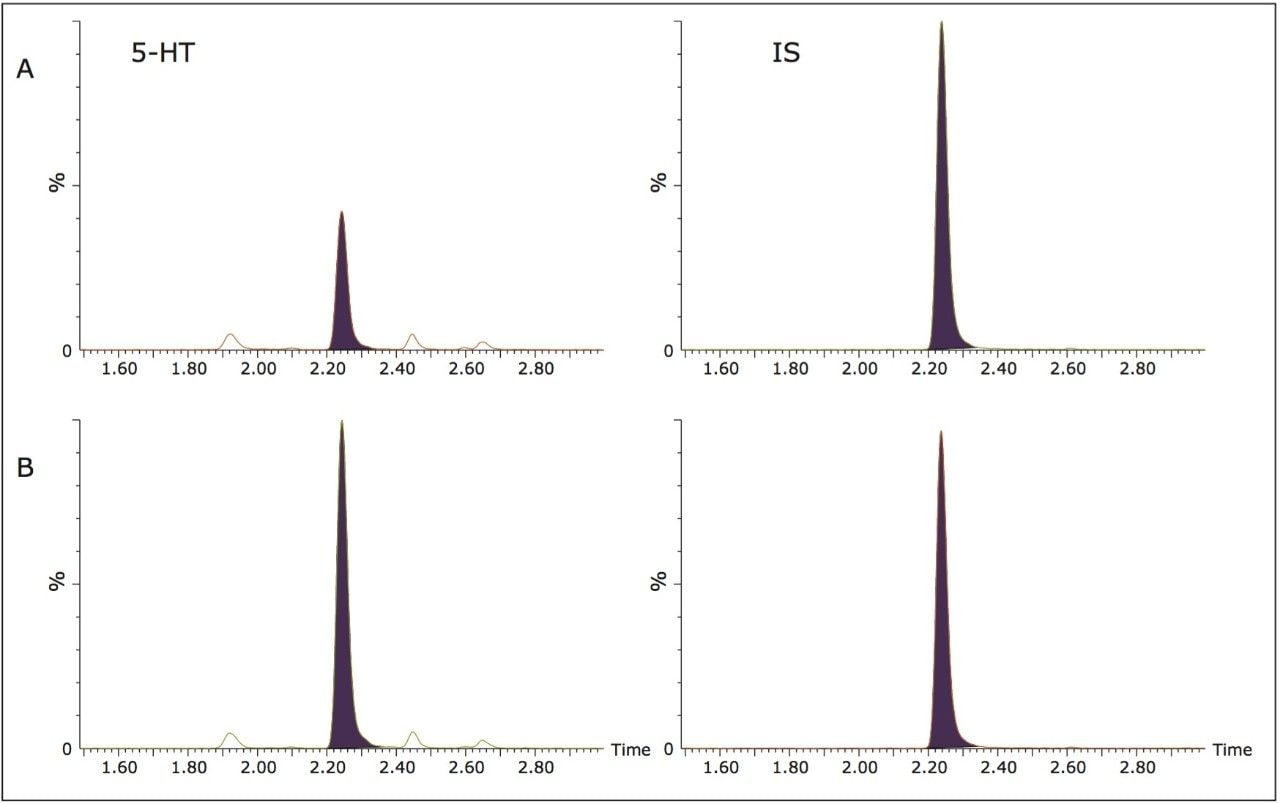
Extraction recovery and matrix effects for urinary serotonin were 80% and -19%, respectively. The unique selectivity provided by the HSS PFP column not only provided the resolution of serotonin from endogenous interfering compounds, but also resulted in minimal matrix effects.
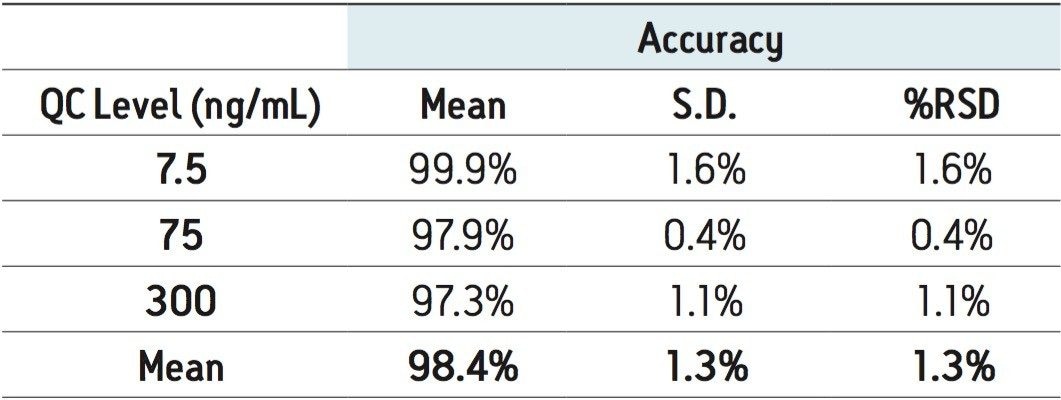
Calibration and quality-control samples were prepared via the standard-addition method by spiking a urine pool with known concentrations of serotonin. After data processing, the endogenous concentration was extrapolated from the resultant calibration curve and the information used to correct the actual calibration and QC concentrations. The urine pool used for calibration was found to contain 29.7-ng/mL of serotonin, so the calibration concentrations were changed from 5.0 to 500 to 34.7 to 529.7 ng/mL. The resultant calibration curve showed excellent linearity, with an R2 value of 0.9997. The quality-control samples (n = 6), over-spiked at 7.5, 75, and 300 ng/mL, demonstrated excellent accuracy and precision (see Table 3). All QC values were within 3% of their target values, and all coefficients of variation were less than 2%. These data demonstrate that the method is linear, accurate, and precise over a measurement range that includes the expected values for normal and elevated urine samples.
The extraction and analysis of urinary serotonin for clinical research using the Oasis, mixed-mode, weak-cation-exchange (WCX) plates and an ACQUITY UPLC HSS PFP Column is detailed. Extraction resulted in low matrix effects and consistent recoveries that translated into excellent analytical precision. The unique selectivity provided by the HSS PFP column resulted in resolution of serotonin from endogenous, potentially interfering compounds and minimal matrix effects. Quantitative results showed a linear response across the measurement range (5 to 500 ng/mL) and excellent accuracy and analytical precision.
720005152, August 2014