In this application, we demonstrate the increased resolution and throughput produced by the Waters ACQUITY UltraPerformance LC (UPLC) System combined with Photodiode Array (PDA) and Waters single quadrupole MS detectors to allow for the identification of more impurities when compared to conventional HPLC.
Impurity profiling of pharmaceutical drug substances or dosage formulations requires methods with high sensitivity, resolution, and throughput. It is a requirement of any regulatory submission that methods are developed not only for the active ingredient, but also for the impurities resulting from the manufacturing process or as a result of product degradation during storage. Ranitidine belongs to a class of drugs called Histamine-2 receptor antagonists (H2-blockers) that prevent or block the production of gastric acid. H2-blockers are broadly used to treat ulcers, gastroesophageal reflux disease (GERD), and conditions where the stomach produces too much acid such as Zollinger-Ellison Syndrome. Ranitidine is specifically used to prevent and treat symptoms of heartburn associated with acid indigestion and sour stomach.
Currently, the assay and purity tests described in the United States Pharmacopoeia (USP) for ranitidine and its related substances employ time-consuming and labor-intensive thin layer chromatography and HPLC methodologies.2,4 A more recent approach uses capillary electrophoresis-based quantification with a 30-minute run time.1 The competitive nature of today's drug development market makes these long run times incompatible with high throughput laboratory workflows.
In this application, we demonstrate the increased resolution and throughput produced by the Waters ACQUITY UltraPerformance LC (UPLC) System combined with Photodiode Array (PDA) and Waters single quadrupole MS detectors to allow for the identification of more impurities when compared to conventional HPLC. Though this technique is application-specific, UPLC with PDA and MS has global potential to fast track method development in today’s pharmaceutical pipeline.
Ideally, authentic impurity standards would be used to prepare the solutions for use in the method development process. However, in many situations, impurity standards are not initially available, and it is therefore necessary to forcefully degrade the drug substance to generate them. In this application, standards were prepared by refluxing ranitidine (2 mg/mL) at 85 °C for 48 hours. LC method selection was performed via traditional approaches of method scouting with four columns at various pHs, and temperature was explored with a goal of maximizing peak resolution and the number of peaks detected while keeping the run time to a minimum. Method optimization was performed with the assistance of method development simulation software, DryLab 2000 Plus (Rheodyne, Rohnert Park, CA). Method development tools such as single quadrupole MS detection for peak identification, and mass spectral/PDA-UV spectral analysis for peak tracking were employed to ensure maximum peak detection and streamline the method development process.
|
LC system: |
ACQUITY UPLC System with the ACQUITY UPLC PDA Detector and Empower Software |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 μm (four columns were explored) |
|
Mobile phase: |
A: 20 mM Ammonium Bicarbonate, pH 9.0 (two pHs were explored) B: Methanol |
|
Weak wash: |
95/5 Water/MeOH, 1200 μL |
|
Strong wash: |
50/50 Water/MeOH, 300 μL |
|
Flow rate: |
0.45 mL/min |
|
Injection volume: |
1.0 μL |
|
Temperature: |
50 °C |
|
UV Detection: |
230 nm |
|
Time (min) |
Profile |
|
|
A% |
%B |
|
|
0.0 |
96.0 |
4.0 |
|
1.0 |
84.0 |
16.0 |
|
4.0 |
64.0 |
36.0 |
|
7.0 |
10.0 |
90.0 |
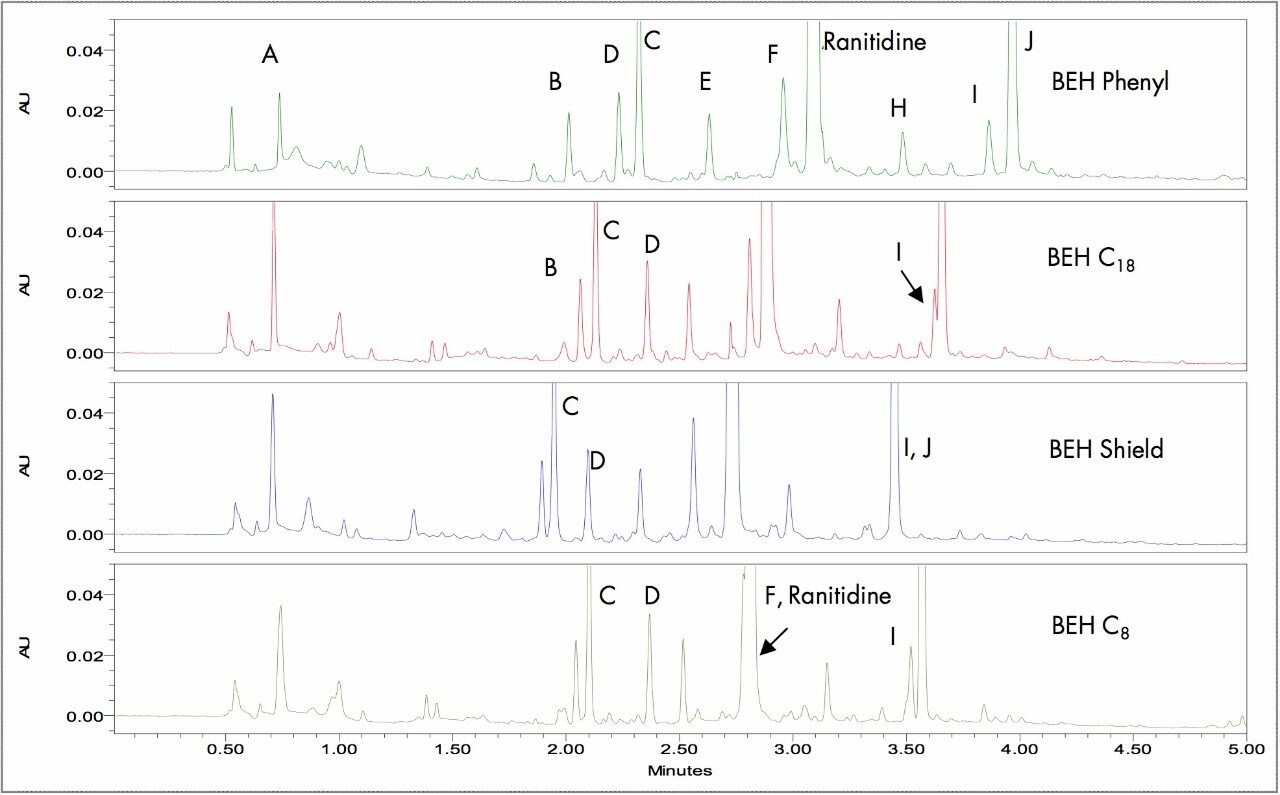
Manipulating SelectivityThe four different column chemistries under study yielded subtle selectivity differences as shown below in Figure 1. The major impurities are labeled A–J. A 5-minute scouting gradient of 5−90 %B methanol with 20 mM ammonium bicarbonate (pH 9.0) was employed for column evaluation. The initial results showed that the phenyl chemistry provided the best resolution of the impurity peaks. However, the BEH C18 Column was selected for method development due to the overall superior peak shapes and sensitivity produced for the impurities. Although the resolution of the I and J peaks was best using the Phenyl Column, the chromatography produced by the BEH C18 Column could be optimized with the simulation software to provide the required resolution.
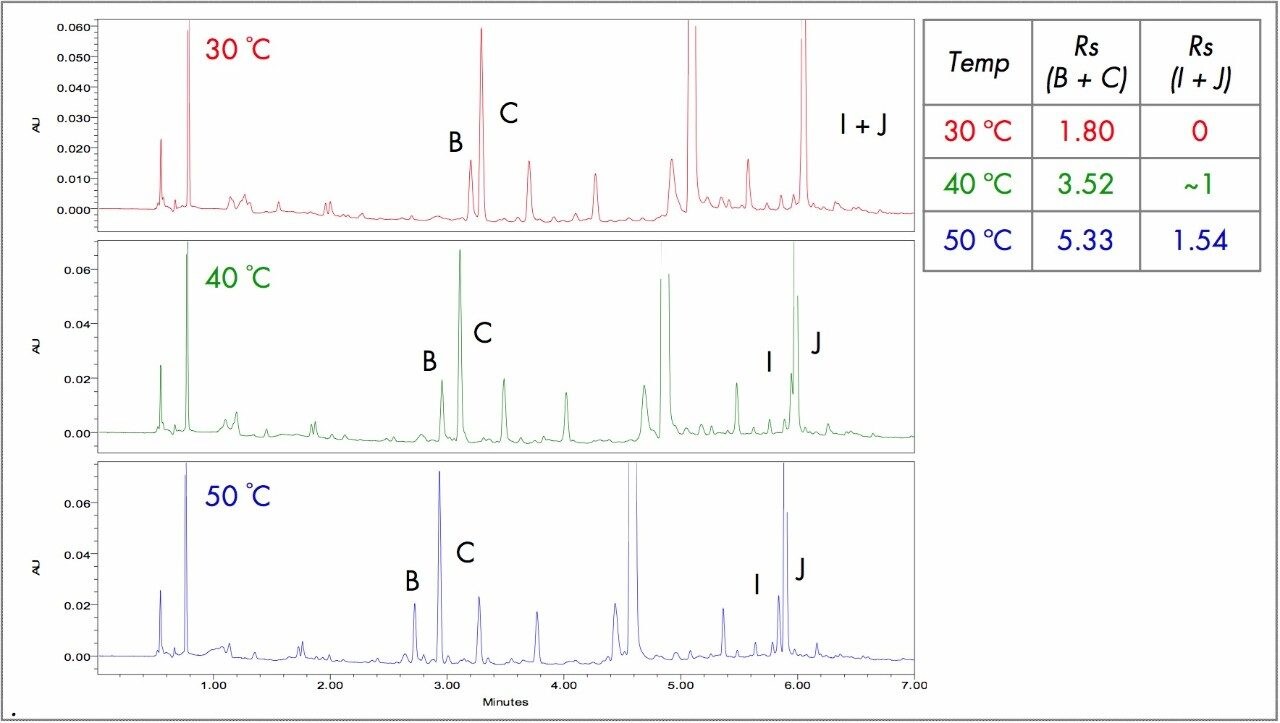
Temperature exploration is an easy and straightforward experiment set to carry out. Increases in tempedecreases analyte retention times, allow for faster flow rates, and shorter run times. It is recommended that temperature is best optimized once the columnchemistry and pH are chosen and/or when all method development options have been exhausted. In Figure 3, we can see that increasing tempera gradient-and pH-optimized method resulted in sharper peaks and increased resolution (R).
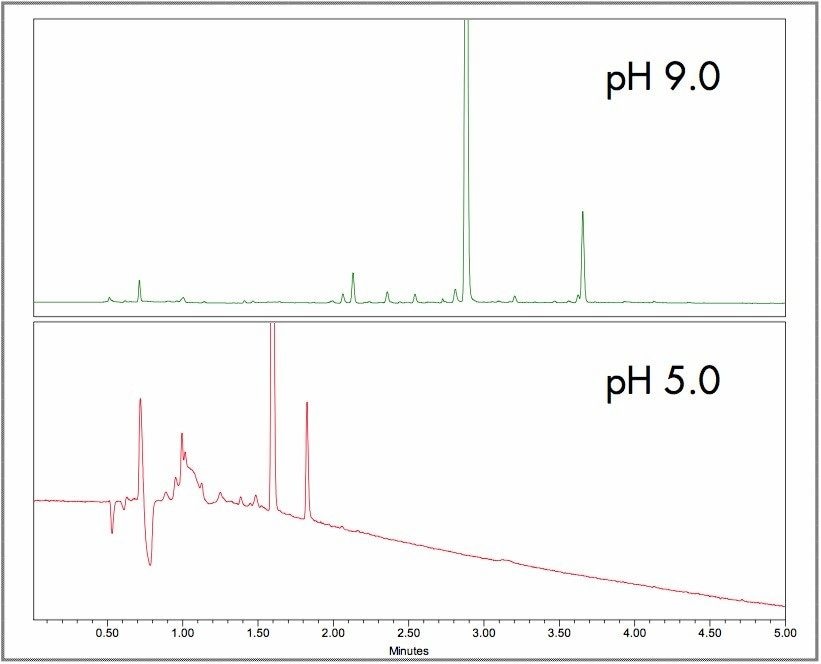
The gradient and pH conditions were optimizeon the results from previous experiments (Figures 1 and 2). The initial conditions started at 4% methanol with a linear gradient to 16% over 1 minute, followed second linear step increase to 36% to 4 minutes and a final gradient linear ramp to 90% organic at 7 minute.
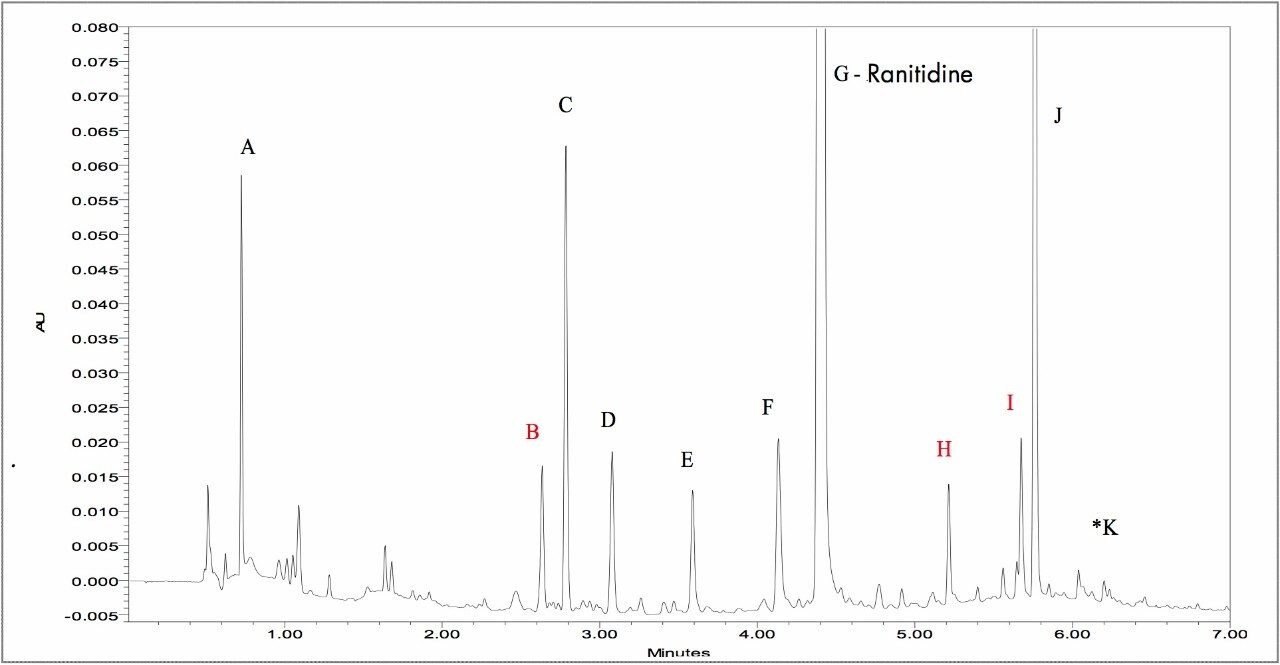
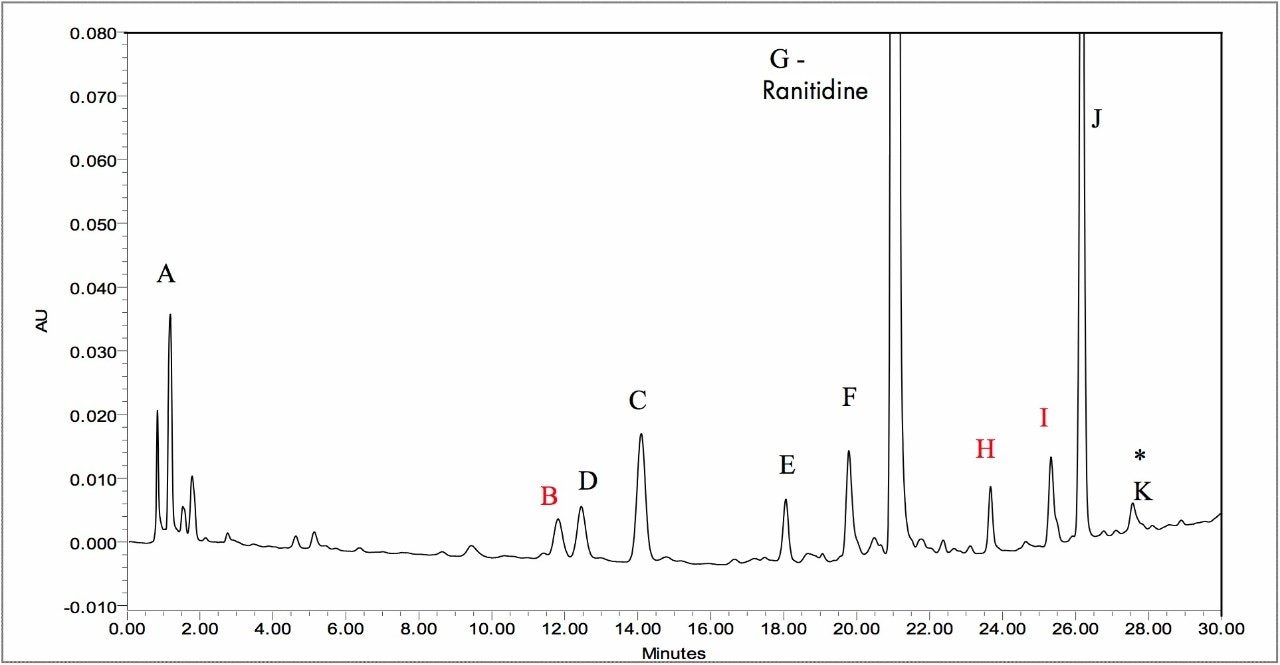
The final method was optimized for maximum separation of peaks and maximum resolution in the shortest possible run time. This method had an analysis time of 6 minutes with all 11 impurity peaks completely resolved. The resulting method maintained the ranitidine peak on-scale without detector saturation, allowing for an increased injection volume or higher sample concentration column load to be used to facilitate the detection of other small impurity/degradant peaks. The increased peak intensity, reduced run time, and increased number of detected peaks produced by the ACQUITY UPLC System is evident when compared to the HPLC separation (Figure 5).
For final method details, please refer to the ‘LC Conditions’ outlined in the Experimental section.
A. 3-(methylamino)-5,6-dihydro-2H-1,4-thiazin-2-one oxime
B. *Unknown m/z = 387.14
C. N-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)sulfinyl]ethyl}-N'-methyl-2-nitro-1,1-ethenediamine
D. {5-[(dimethylamino)methyl]-2-furyl}methanol (M+H=156.1024)
E. N-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)sulfanyl]ethyl}-2-nitroacetamide
F. 2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)sulfanyl]ethanamine
G. Ranitidine (active)
H. *Unknown m/z = 284.11
I. *Unknown m/z = 297.16
J. Dimer(reported as Formaldehyde adduct)2
K. N,N'-bis[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-2-nitro-1,1-ethenediamine
*Unknown components based on current MS data.3
The Waters single quadrupole mass detector was configured in-line with the PDA detector for peak tracking and preliminary peak identification confirmation. PEEK tubing (0.005-inch i.d.) was cut to minimal length to minimize band broadening when using the two detectors in series. Empower Software allows for the rapid review of peak integration and MS spectra, along with extracted ion chromatograms, for simplified peak matching.
Optimization using chromatography simulation software can expedite much of the development process. As this software is entirely dependent on the quality of data input, tracking and properly matching both resolved and co-eluting peaks from each injection using the MS data lead to more successful predictions. DryLab was used to assist the method optimization of this ranitidine example (not shown). The gradient editor tool was used to optimize the conditions when no single linear options met the resolution goals.
An example analysis utilizing an HPLC method is provided in Figure 5. Differences in column chemistry properties make it difficult to show true, scaled comparisons with no differences in selectivity. However, injection volume scaling calculations were performed to maintain proper column load integrity.
|
LC system: |
Alliance System with the 2996 PDA Detector and Empower Software |
|
|
Column: |
XTerra MS C18 3.9 x 150 mm, 5 μm |
|
|
Mobile phase: |
A: 20 mM Ammonium Bicarbonate, pH 9.0 B: Methanol |
|
|
Flow rate: |
1.5 mL/min |
|
|
Injection volume: |
5.0 μL |
|
|
Temperature: |
50 °C |
|
|
UV Detection: |
230 nm |
|
Time(min) |
Profile |
|
|
%A |
%B |
|
|
0.0 |
95.0 |
5.0 |
|
14.0 |
86.0 |
14.0 |
|
30.0 |
35.0 |
65.0 |
To achieve the same resolution between any critical pair of peaks with HPLC analyses compared to that of UPLC resulted in a ~30-minute run time vs. a 7-minute run time, respectively. The peaks produced by the UPLC column were sharper and more intense, resulting in a more sensitive assay. Comparison of the UV signal-to-noise ratios between HPLC and UPLC yielded an average 4.6-fold increase (median 2.9-fold increase), while sample load remained constant. The combined resolution and sensitivity benefits of ACQUITY UPLC facilitated the detection of 45 peaks in the sample with a peak area greater or equal to 0.05% of that of ranitidine, whereas with HPLC only 34 peaks were detected at the same level.
As demonstrated, the ACQUITY UltraPerformance LC System can help in fast-tracking method development of purity analysis methods. UPLC decreased analysis run times by 4.2-fold when compared to that of the USP-published HPLC or CE methods,1 while increasing sensitivity and maintaining resolution between ranitidine and its impurities. Traditional chromatographic principles are adhered to by UPLC and thus the established method development approaches of column scouting and pH variation can be employed. UPLC is readily compatible with many of the HPLC method development tools used today such as simulation software, PDA, and single quadrupole MS for peak tracking.
720001468, April 2006