This application note describes the analysis of beclomethasone using the Waters Micromass Quattro micro API bench top Tandem Quadrupole MS, incorporating the ZSpray interface.
Demonstrates the robustness of the IonSABRE interface and ZSpray source for analyses from complex matrices
Many compounds analyzed in the pharmaceutical arena are assayed in complex matrices. Atmospheric pressure chemical ionization (APCI) allows for sensitive analysis of compounds in protein precipitated plasma. LC-MS/MS analysis is a vital aspect of the drug development process within the pharmaceutical industry. The IonSABRE is a new APCI probe that has been developed with thermally balanced vaporization to optimize heat distribution. The experiment outlined below was designed to demonstrate the robustness of the IonSABRE probe with protein-precipitated plasma samples, in this case for the analysis of beclomethasone using the Waters Micromass Quattro micro API bench top Tandem Quadrupole MS, incorporating the ZSpray interface.

A quality control (QC) solution of beclomethasone in human plasma was prepared at a concentration of 1000 pg/mL and 250 repeat injections were performed.
A calibration series of beclomethasone in human plasma was prepared over the concentration range of 50–50000 pg/mL and the peak area plotted against concentration.
The calibration series and QC were protein-precipitated by adding acetonitrile. The resultant mixture was centrifuged (ca 3000 rpm, 10 mins) and the supernatant taken for analysis by LC-MS/MS.
|
LC system: |
Waters Alliance 2695 |
|
Column: |
Waters XTerra MS, C18 3.5 μm, 4.6 x 50 mm |
|
Flow rate: |
1 mL/min |
|
Gradient: |
Isocratic at 50% water:50% acetonitrile |
|
Ion mode: |
APCI +ve (IonSABRE) |
|
Cone voltage: |
25 V |
|
Collision energy: |
25 eV |
|
Detection mode: |
MRM (369.3 > 147.1) m/z |
|
Dwell: |
0.5 seconds |
|
Collision gas: |
Argon (5 x 10-3 mbar) |
The MRM chromatograms for the beclomethasone analysis were integrated automatically using ApexTrack and the resulting peak areas were plotted against injection number to show the reproducibility of response over the analysis period (see Figure 1).
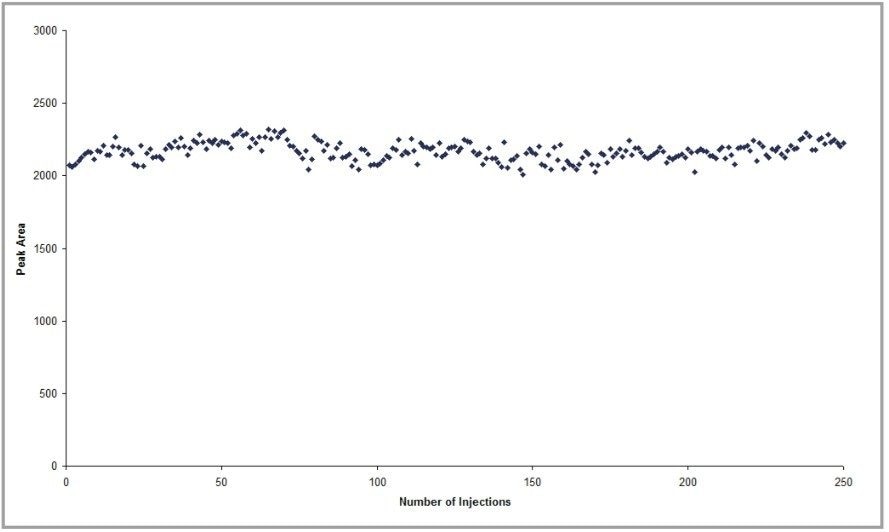
Figure 2 shows the chromatograms for samples 50, 150, and 250. The average area response was 2168 with a relative standard deviation of 2.9% across all 250 analyses.

In this experiment, protein-precipitated plasma, a complex matrix containing involatile compounds, was sprayed into the source for approximately 24 hours, without any reduction in response. This removes the need for time-consuming sample preparation and maximizes uptime of the system.
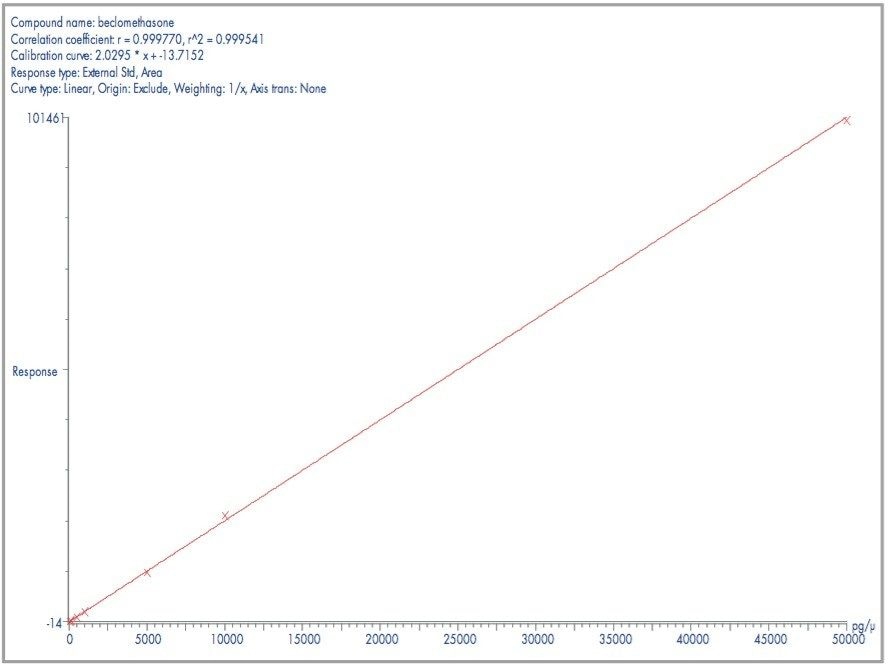
The plot of peak area against concentration showed good linearity over the range 50–50000 pg/mL. The calibration line was plotted using a linear fit with 1/x weighting and gave a coefficient of determination of 0.9995, with all calibration points resulting in <5% deviation (see Figure 3).
The Waters Micromass Quattro micro API Tandem Quadrupole Mass Spectrometer has been developed for quantitative LC-MS/MS. The robustness of the IonSABRE to biological matrices has been evaluated by analyzing beclomethasone in blank human plasma.
The results show that the sensitivity was maintained with a relative standard deviation of 2.9% during 250 injections of beclomethasone in proteinprecipitated plasma. This demonstrates the robustness of the IonSABRE interface and ZSpray source for analyses from complex matrices. The calibration line resulted in a linear plot over the range 50 to 50000 pg/μL with a correlation coefficient of 0.9995.
720000977, November 2004