Advancing Charge Variant Analysis with pH-Gradient Ion-Exchange Chromatography: Optimizing Monoclonal Antibody Characterization Using the High-pH Kit for the Alliance™ iS Bio HPLC System
Corey E. Reed, Jennifer Simeone, Paula Hong
Waters Corporation, United States
Published on August 04, 2025
Abstract
Charge variant analysis is a critical component in the characterization of monoclonal antibody (mAb) therapeutics, ensuring product consistency, safety, and efficacy. This study employs a pH-gradient ion-exchange chromatography method to evaluate charge heterogeneity using the Alliance iS Bio HPLC System equipped with a high-pH kit. Two high-pH kits were assessed for suitability, demonstrating exceptional repeatability and reproducibility when analyzing a Waters™ mAb Charge Variant Standard. Peak area and retention time %RSDs consistently met acceptance criteria, confirming robust performance.
Additionally, the method was applied to infliximab charge variant analysis, revealing precise quantification of lysine charge variants. The high-pH kit was installed on the Alliance iS Bio HPLC System and coupled with both the BioResolve™ SCX mAb Column and BioResolve CX pH Concentrates for a pH gradient ion-exchange separation. Testing of the mAb sample showed good separation of the lysine variants. Furthermore, the high-pH kits produced reproducible results, with relative peak area variations within 3–5%, highlighting method reliability. Minor charge variants were consistently detected, underscoring the analytical sensitivity of the approach. Importantly, despite operating under extreme pH conditions, system performance remained stable over time, mitigating concerns of instrument degradation.
The results validate the Alliance iS Bio HPLC System with high-pH enhancements as a powerful tool for routine charge variant monitoring with a pH gradient in biopharmaceutical development. The study establishes a robust, sensitive, and reproducible method, ensuring confidence in charge variant characterization for therapeutic mAbs.
Benefits
- The Alliance iS Bio HPLC System with a high-pH kit provides a system compatible with a pH gradient ion-exchange separation for lysine variants of mAb
- The high-pH kit enabled stable performance of the Alliance iS Bio HPLC System under extreme pH conditions to ensure long-term reliability
Introduction
Lysine charge variant analysis is a critical quality attribute in the characterization of mAb therapeutics, playing a pivotal role in ensuring the safety, efficacy, and consistency of drug products. Charge heterogeneity, particularly at the C-terminal lysine, can arise due to manufacturing conditions, post-translational modifications, or protein degradation, potentially impacting the drug’s biological activity and immunogenicity.1 Understanding and accurately quantifying these charge variants is essential for regulatory compliance, batch comparability, and maintaining therapeutic integrity.
Charge variant analysis serves multiple functions beyond routine quality control. It enables comparability assessments between drug product batches, helping to detect subtle manufacturing changes, and facilitates biosimilar development by evaluating structural and functional similarity to the originator biologic. Since C-terminal lysine modifications induce a slight shift in the molecule’s isoelectric point (pI), this variation can be leveraged through pH-gradient ion-exchange chromatography (IEX) to achieve separation of charge variants. As analytes traverse the chromatographic column, they elute according to the order of their pI values as determined by the overall charge state at the surface of the molecule, providing a detailed resolution of molecular heterogeneity.
While pH-gradient IEX is a powerful technique for charge variant analysis, instrument compatibility remains a critical factor in ensuring reliable and reproducible results. Traditional high-performance liquid chromatography (HPLC) systems can be vulnerable to corrosion at extreme pH levels, potentially affecting performance and longevity. To mitigate these concerns, Waters has developed a high-pH kit tailored for use with the Alliance iS Bio HPLC System providing enhanced durability and analytical precision.
The high-pH kit includes a specialized sample needle cartridge assembly, extension loop, needle seal, seal port tubing, preheater, and TUV flow cell, all constructed from corrosion-resistant MP35N alloy to withstand prolonged exposure to elevated pH conditions. The Alliance iS Bio HPLC System quaternary pump also utilizes corrosion resistant materials such as PEEK, titanium, and MP35N throughout. This robust design ensures system stability while maintaining method accuracy and reproducibility. Specifically engineered for analytical workflows operating above pH 10.0 and below pH 13.0, the kit enables the reliable execution of high-pH chromatographic methods, ensuring consistent charge variant characterization without compromising instrument integrity.
In this study, a pH-gradient IEX method is employed on an Alliance iS Bio HPLC System equipped with 2 high-pH kits, to assess system suitability and ensure consistency in charge variant characterization. Infliximab, a therapeutic mAb, is analyzed to quantify its lysine charge variants, demonstrating the effectiveness of this approach in monitoring biopharmaceutical quality.
Experimental
Sample Description
Waters mAb Charge Variant Standard (p/n: 186009065) was reconstituted to a final concentration of 900 µg/mL with 100 µL of water and vortexed. Remicade® (infliximab) was diluted to a final concentration of 10 mg/mL with water and analyzed past expiry.
Method Conditions
LC Conditions
|
LC system: |
Alliance iS Bio HPLC System with High-pH kit (p/n: 205002588) |
|
Detection: |
TUV, 280 nm @ 10 Hz |
|
Column(s): |
BioResolve SCX mAb Column, 3 µm, 4.6 x 50 mm (p/n: 186009058) |
|
Column temperature: |
30 °C |
|
Sample temperature: |
8 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
1.44 mL/min |
|
Mobile phase A: |
A 10:1 dilution of BioResolve CX pH Concentrate A, pH 5.0 (p/n: 186009063) |
|
Mobile phase B: |
A 10:1 dilution of BioResolve CX pH Concentrate B, pH 10.2 (p/n: 186009064) |
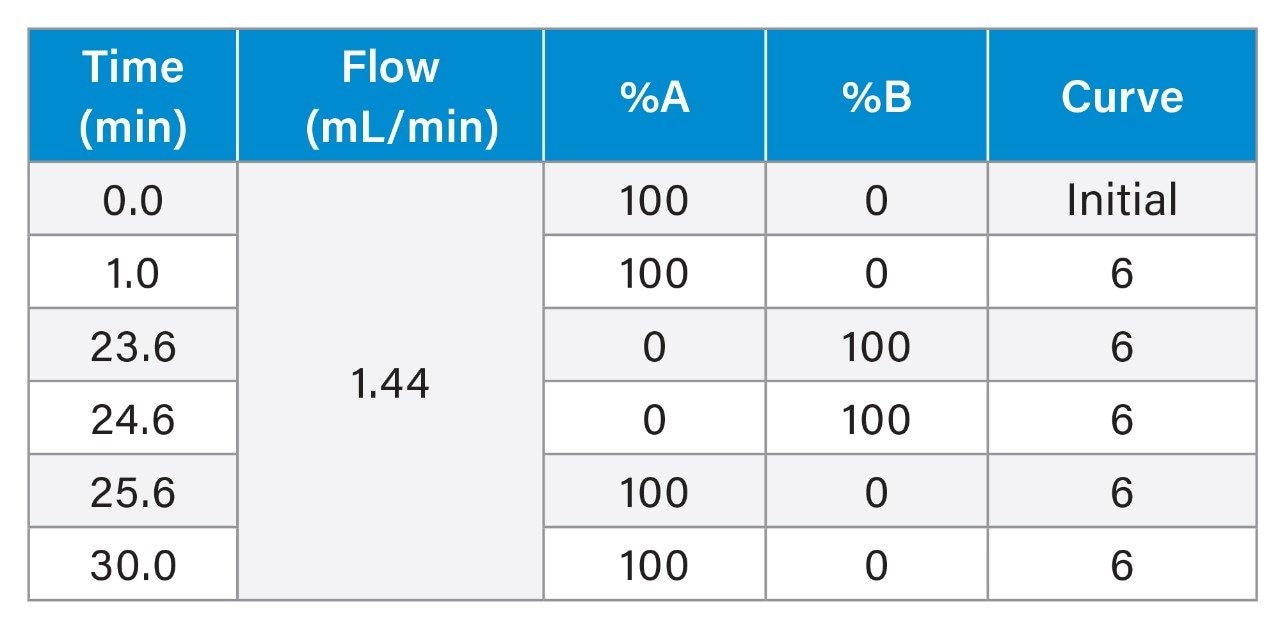
Gradient Table
Data Management
|
Chromatography software: |
Empower™ Chromatography Data System |
Results and Discussion
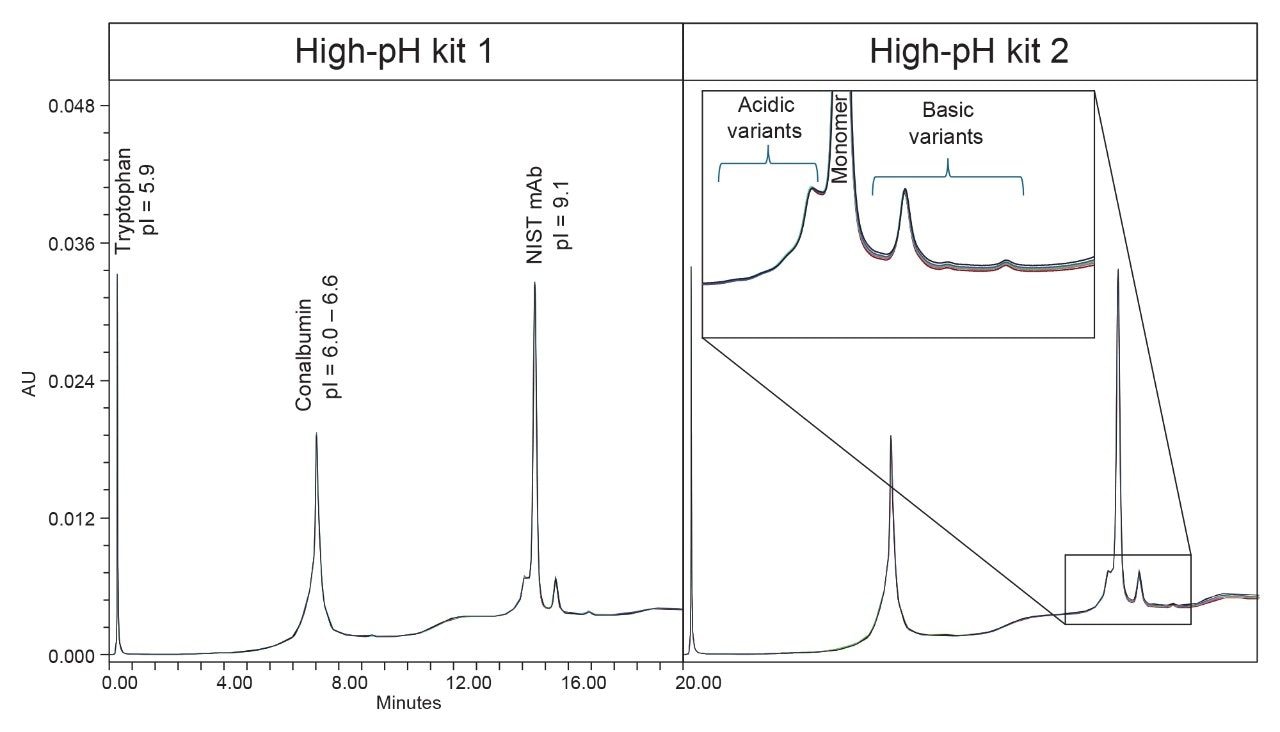
The pH-gradient employed in this study transitions from pH 5.0 to pH 10.2 over a 22.6-minute period, facilitating the precise separation of charge variants based on their pI. As the gradient progresses, it shifts from an acidic starting condition through neutral pH, ultimately reaching basic conditions. This controlled pH transition ensures that each component in the Waters mAb Charge Variant Standard elutes sequentially according to its increasing pI, providing a well-defined resolution between molecules. Overlaid chromatograms using 2 high-pH kits on the same system are depicted in Figure 1 along with the pI of each molecule.
Critically, this method demonstrates exceptional sensitivity, allowing for the separation of heterogeneous variants of a single molecule. This is exemplified by the NIST mAb peak, where acidic variants elute before the monomer at lower pH, while basic variants emerge following the monomer peak. Such resolution is particularly valuable in infliximab charge variant analysis, as demonstrated in later sections, where subtle charge differences must be accurately identified to ensure product consistency and therapeutic integrity.
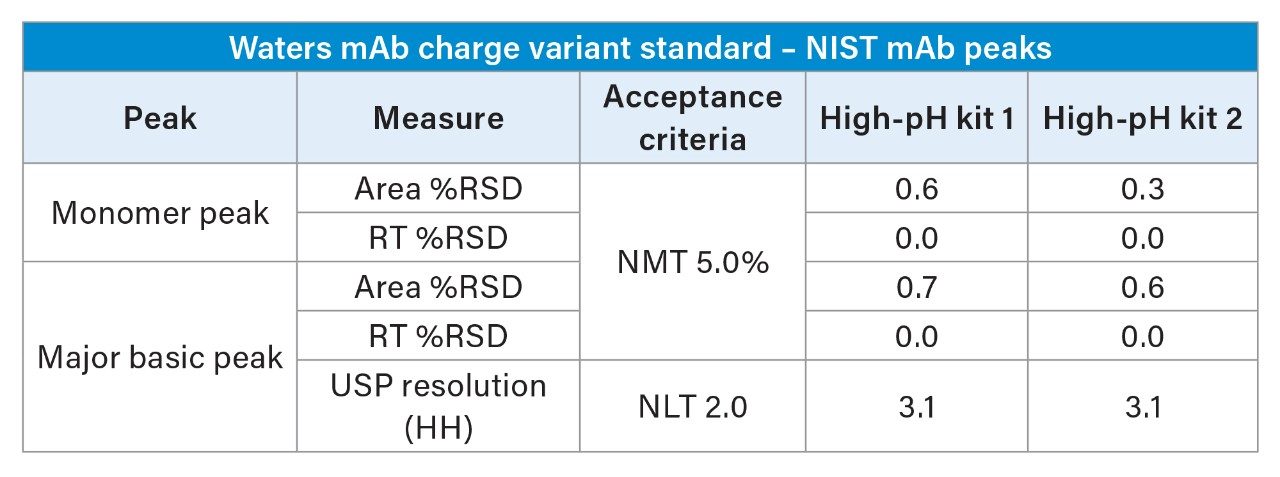
To assess analytical robustness and reproducibility, six replicate injections of Waters mAb Charge Variant Standard were analyzed on each system. These injections exhibited high precision, reinforcing the method's reliability across multiple runs. The assay’s acceptance criteria, outlined in Table I, set stringent performance benchmarks. Both instruments, equipped with high-pH kits, consistently met these criteria, demonstrating strong system suitability and stable method performance. The integration of these high-pH kits not only protects instrument longevity but also delivers high quality and repeatable data, ensuring confidence in the accuracy of charge variant characterization.
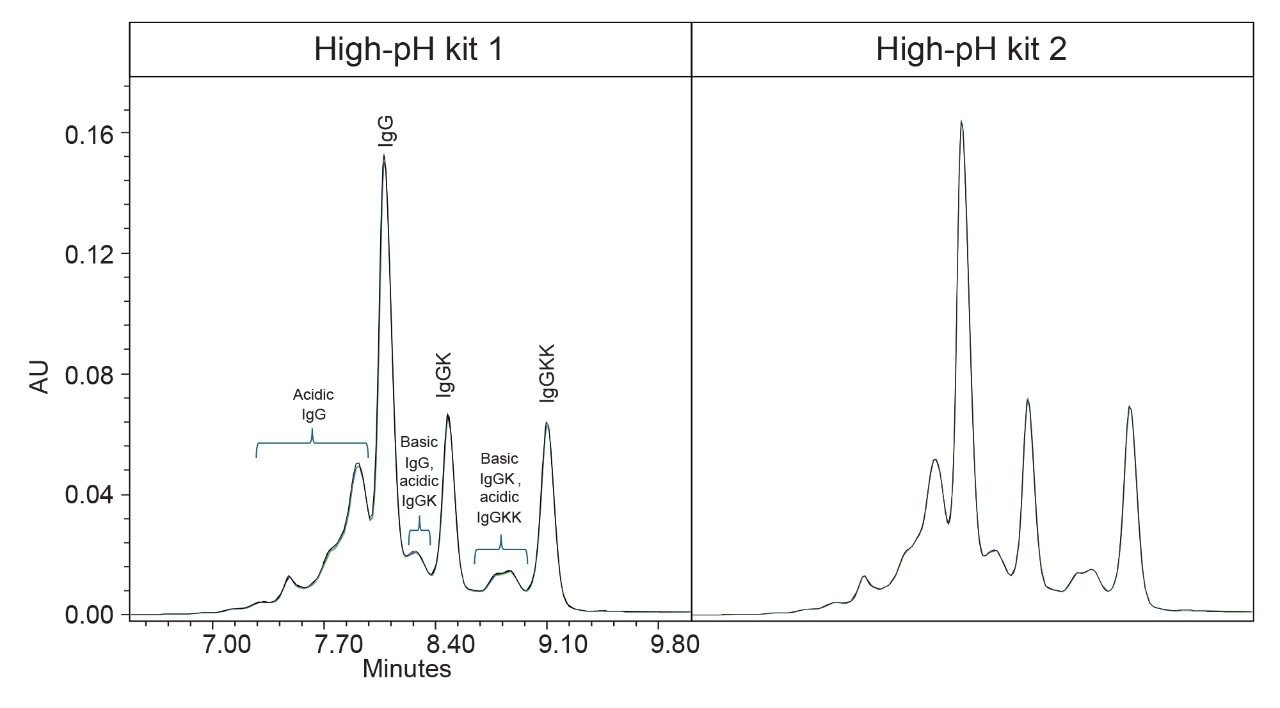
As previously discussed, method sensitivity is a key factor in the charge variant analysis of mAb samples given its inherent complexity. These biologics often exhibit a high degree of heterogeneity, with numerous co-eluting peaks that challenge resolution and quantification. A robust analytical method must not only distinguish subtle charge variants but also maintain high precision and repeatability.
Figure 2 presents the overlay of three replicate injections of infliximab analyzed using both high-pH kits. As observed with the Waters mAb Charge Variant Standard, the instrument demonstrates strong performance using both kits, ensuring confidence in data consistency. The alignment of chromatographic profiles visually confirms excellent repeatability, reinforcing the method's reliability in detecting minor charge variants while maintaining peak integrity.
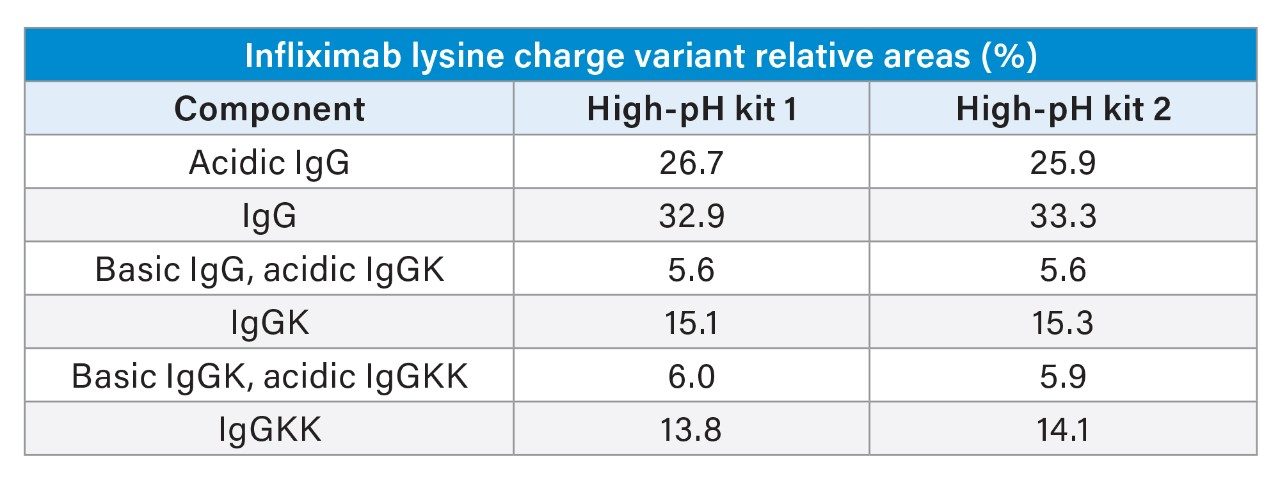
Further supporting hardware kit reproducibility, Table II provides a quantitative comparison of relative peak areas of the infliximab charge variants. Major lysine charge variant peaks (IgG, IgGK, and IgGKK) are within 0.4% relative area as compared kit-to-kit while the minor charge isoforms remain within 0.8%. These findings validate the suitability of the Alliance iS Bio HPLC System with high-pH kit for routine monitoring and comparability studies, offering a reliable framework for monitoring batch consistency in therapeutic mAb production.
Conclusion
The Alliance iS Bio HPLC System equipped with a high-pH kit and combined with the BioResolve SCX mAb Column and BioResolve CX Concentrates demonstrated exceptional performance for a pH-gradient IEX method of mAb charge variants. To assess system suitability, two independent high-pH kits were evaluated using a Waters mAb Charge Variant Standard, ensuring consistency and reliability of the methodology. With six replicate injections, the system achieved peak area %RSDs of ≤ 0.7% for the monomer and major basic peak of the NIST mAb component, with retention times %RSDs of 0.0%, far exceeding the acceptance criteria of ≤ 5.0% for both parameters. Additionally, resolution between the monomer and major basic peak met the criteria of ≥ 2.0, with both high-pH kits averaging 3.1 across the 6 replicates, demonstrating robust separation capabilities.
Further validation of system suitability and analytical precision was confirmed in the charge variant analysis of infliximab, a widely used therapeutic mAb. Triplicate injections performed on each system showed strong agreement in average relative peak areas, with average major lysine charge variants differing by no more than 0.4% between kits, while acidic and basic isoforms exhibited variability within 0.8% relative area. These findings underscore the excellent reproducibility and repeatability of the method, reinforcing its capability for reliable determination of charge variants. Additionally, minor charge variants of both NIST mAb and infliximab were consistently detected, confirming the method’s sensitivity in resolving subtle molecular heterogeneity.
Importantly, system performance remained stable over time, despite the extreme pH fluctuations inherent to pH-gradient IEX chromatography, mitigating concerns about instrument degradation and ensuring long-term reliability. These results confirm that the Alliance iS Bio HPLC System, integrated with the MP35N high-pH kit, provides excellent assay sensitivity, precision, and robustness, making it an ideal platform for charge variant analysis in biopharmaceutical development and characterization.
References
- Dick, L. W. J.; Qui, D.; Mahon, D.; Adamo, M.; Cheng, K.-C. C-Terminal Lysine Variants in Fully Homan Monoclonal Antibodies: Investigation of Test Methods and Possible Causes. Biotechnol. Bioeng. 2008, 100 (6), 1132–1143. https://doi.org/10.1002/bit.21855.
720008968, July 2025