Illustration of the Analytical Performance of the Waters MassTrak Endocrine Steroid Calibrators and QC Set Using the ACQUITY UPLC I-Class and Xevo TQ-S micro Mass Spectrometer
This is an Application Brief and does not contain a detailed Experimental section.
For in vitro diagnostic use. Not available in all countries.
Abstract
This document describes a test of the analytical performance of the MassTrak Endocrine Steroid Calibrator and QC Set (IVD) using the Waters ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System.
Introduction
The Waters MassTrak Endocrine Steroid Calibrator and QC Set (IVD) contains metrological traceable steroid hormones in lyophilized human serum, which can be used for quantification of DHEA-S, cortisol, 21-deoxycortisol, corticosterone, 11-deoxycortisol, androstenedione, 11-deoxycorticosterone, testosterone, DHEA, 17-OHP, DHT, and progesterone. The Waters ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System enables the quantification of compounds in biological matrices.
Experimental
The ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System was controlled by MassLynx IVD Software (v4.2) and the data processed using the TargetLynx XS Application Manager. Calibrators and Quality Controls were prepared by reconstituting the MassTrak Endocrine Steroid Calibrator and QC Set (IVD) following the Instructions for Use (IFU) and the samples were processed using the following conditions:
Sample Preparation Conditions
125 µL sample was processed with internal standard, methanol, diluted with water and centrifuged. An aliquot of each sample was transferred to the collection plate and the remaining sample was loaded onto Oasis MAX µElution Plates, washed and eluted into the collection plate prior to analysis.
Liquid Chromatography Conditions
|
Column: |
CORTECS C8, 2.1 mm x 100 mm, 2.6 µm with VanGuard Pre-Column |
|
Mobile phase A: |
0.1 mM Ammonium fluoride in water |
|
Mobile phase B: |
0.1 mM Ammonium fluoride in methanol |
|
Flow rate: |
0.3 mL/min |
|
Gradient: |
40% B over 1.25 minutes, 40–52.5% B over 1.75 minutes, 52.5% B for 1 minutes, 52.5%–65% B over 1 minutes, 65% B for 1.25 minutes, 95% B for 0.75 minutes |
Mass Spectrometry Conditions
|
Resolution: |
MS1 (0.75 FWHM), MS2 (0.5 FWHM) |
|
Acquisition mode: |
MRM |
|
Polarity: |
ESI (+/-) |
Results and Discussion
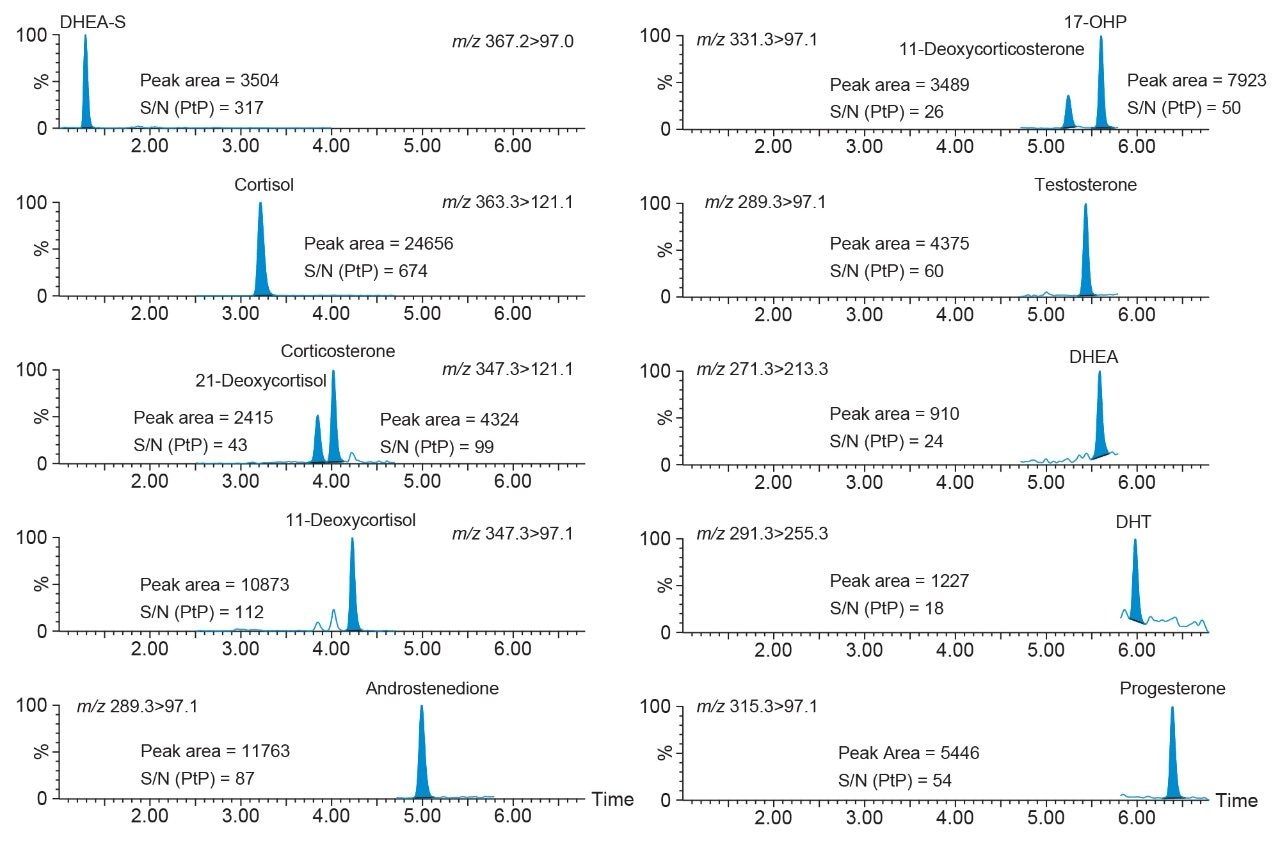
Performance characteristics of the steroid hormones on the Waters ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System are shown in Table 1. Analytical sensitivity of the system for analysing extracted steroid hormone samples is illustrated in Figure 1.
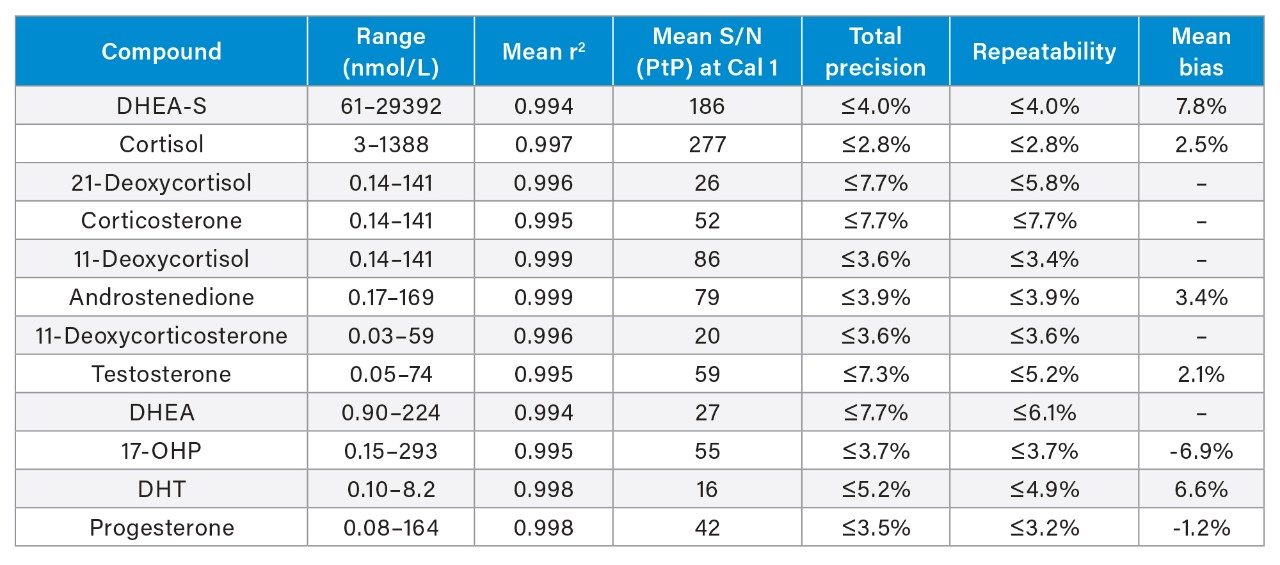
Note: To convert SI units to conventional mass units divide by 2.76 for cortisol (nmol/L to ng/mL), 2.71 for DHEA-S (nmol/L to ng/mL), 2.89 for 21-deoxycortisol, corticosterone, and 11-deoxycortisol (nmol/L to ng/mL), 3.49 for androstenedione (nmol/L to ng/mL), 3.03 for 11-deoxycorticosterone and 17-OHP (nmol/L to ng/mL), 3.47 for testosterone (nmol/L to ng/mL), 3.47 for DHEA (nmol/L to ng/mL, 3.45 for DHT (nmol/L to ng/mL), and 3.18 for progesterone (nmol/L to ng/mL).
Conclusion
The Waters MassTrak Endocrine Steroid Calibrators and QC Set (IVD) in combination with the Waters ACQUITY UPLC I-Class/Xevo TQ-S micro IVD System has demonstrated the capability to deliver analytically sensitive and selective performance with excellent precision and accuracy for DHEA-S, cortisol, 21-deoxycortisol, corticosterone, 11-deoxycortisol, androstenedione, 11-deoxycorticosterone, testosterone, DHEA, 17-OHP, DHT, and progesterone in serum.
Disclaimer
The analytical performance data presented here is for illustrative purposes only. Waters does not recommend or suggest analysis of the analytes described herein. These data are intended solely to demonstrate the performance capabilities of the system for analytes representative of those commonly analyzed using liquid chromatography and tandem mass spectrometry. Performance in an individual laboratory may differ due to a number of factors, including laboratory methods, materials used, intra-operator technique, and system conditions. This document does not constitute a warranty of merchantability or fitness for any particular purpose, express or implied, including for the testing of the analytes in this analysis.
720007372, September 2021