In this application, a comparison is performed between Ostro Pass-through Sample Preparation and SSLE. Typical samples used in drug discovery and development with different acidic, basic and neutral properties and hydrophobicities were analyzed in this evaluation. The mechanisms behind these two techniques and their effects on performance are discussed as well. Ostro shows very high and consistent recoveries across all the tested analytes (>80% with an average RSD <2%). For SSLE lower recoveries were observed for polar analytes (as low as 60% with an average RSD ~8%).
The Ostro Pass-through 96-well plate provides a novel solution for simple pass through cleanup of plasma, serum, and whole blood samples that removes protein and phospholipids with little-to-no method development. It can be quickly implemented into routine lab workflows and produces clean extracts and excellent reproducibility resulting in consistent, robust methods with high throughput. Solid supported liquid extraction (SSLE) is analogous to traditional liquid-liquid extraction (LLE) and utilizes the same water-immiscible solvent systems for analyte extraction. Instead of shaking the two immiscible phases together, in SSLE, the aqueous sample is immobilized on an inert support (diatomaceous earth, a natural siliceous product), and the organic phase flows through the support.
In this application, a comparison is performed between Ostro Pass-through Sample Preparation and SSLE. Typical samples used in drug discovery and development with different acidic, basic and neutral properties and hydrophobicities were analyzed in this evaluation. Key areas of comparison are procedure simplicity, analyte recoveries, matrix factor (MF), and batch-to-batch reproducibility. The mechanisms behind these two techniques and their effects on performance are discussed as well. Ostro shows very high and consistent recoveries across all the tested analytes (>80% with an average RSD <2%). For SSLE lower recoveries were observed for polar analytes (as low as 60% with an average RSD ~8%). For Ostro, excellent batch-to-batch reproducibility was observed. The average % RSD across all the tested batches is 4% and 15% for SSLE. Comparable matrix factors were obtained between Ostro and SSLE, and tips to improve matrix factors for Ostro are presented.
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC CSH C18, 2.1 x 100 mm, 1.7 μm (p/n 186005297) |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Flow rate: |
500 μL/min |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Strong needle wash: |
70/30 ACN/water with 2% formic acid |
|
Weak needle wash: |
70/30 ACN/water with 2% formic acid |
|
Injection mode: |
Partial loop with needle overfill |
|
Injection volume: |
1 μL |
|
Time(min) |
Profile |
Curve |
|
|
%A |
%B |
||
|
0 |
80 |
20 |
- |
|
0.3 |
80 |
20 |
6 |
|
3 |
70 |
30 |
6 |
|
6.5 |
30 |
70 |
6 |
|
6.6 |
80 |
20 |
6 |
|
7 |
80 |
20 |
6 |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
2.5 kV |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
150 L/Hr |
|
Desolvation gas flow: |
1000 L/Hr |
|
MRM transition monitored: |
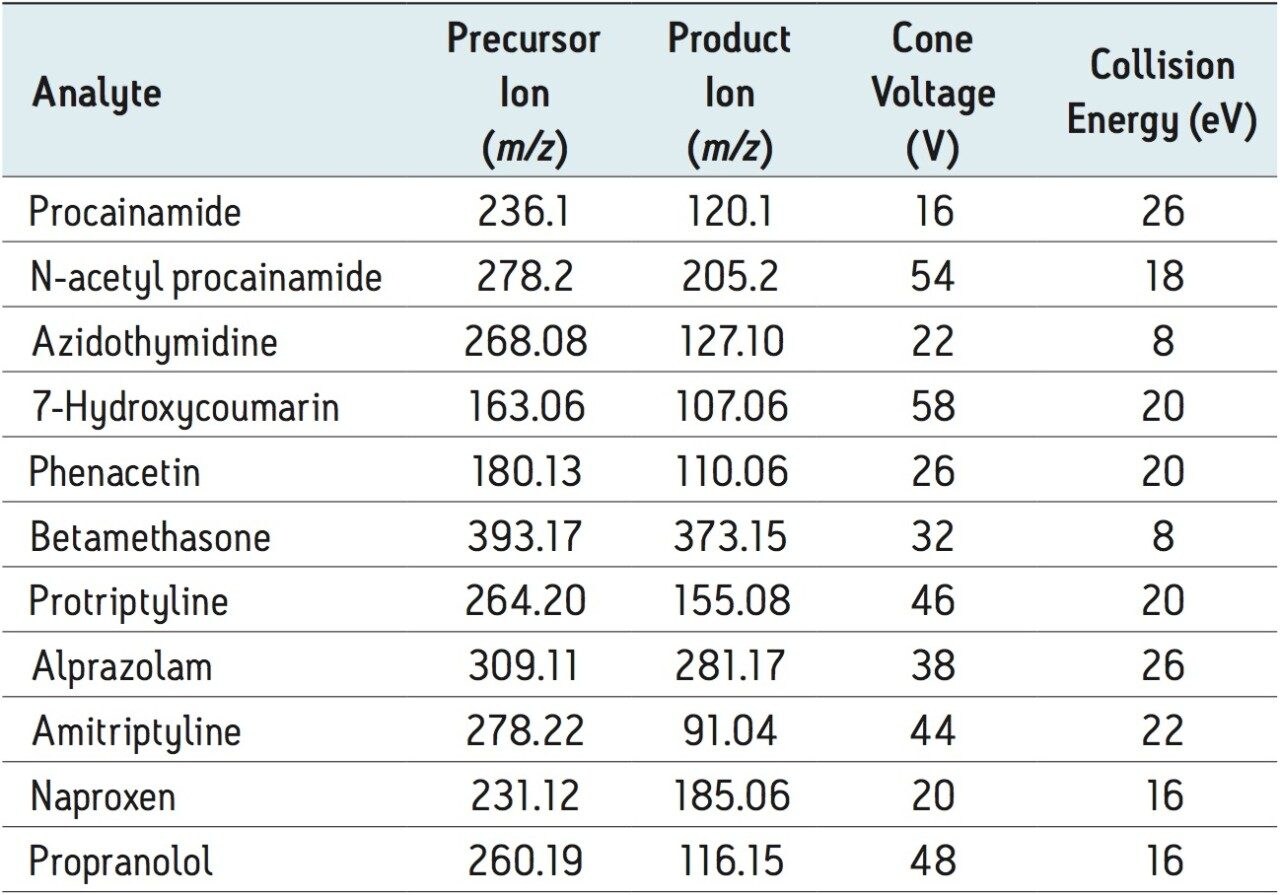
See Table 2 |
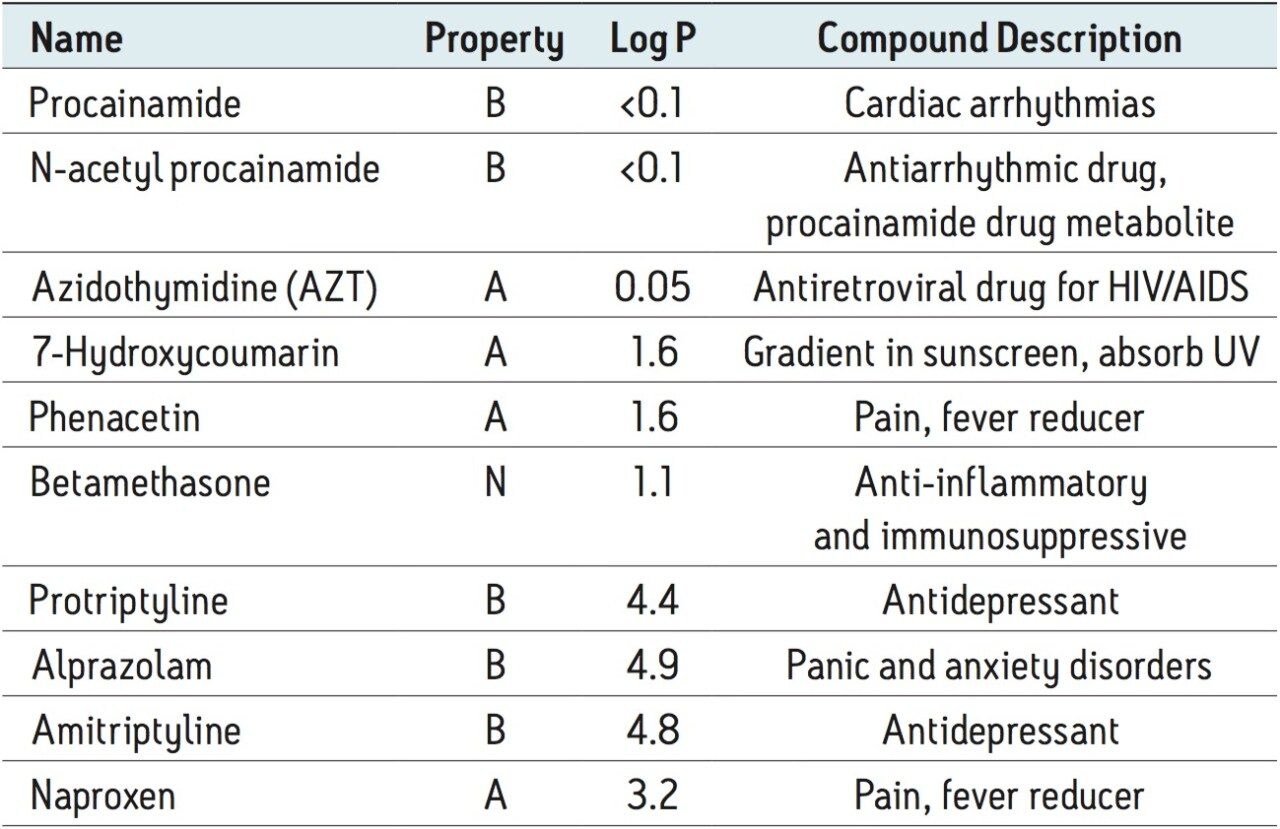
Table 1 lists the tested analytes with their acidic (A), basic (B) or neutral (N) properties, logPs (partition coefficient, indication of hydrophobicity), and compound description. Stock solutions were prepared in 40% acetonitrile. The concentration for stock solutions is 0.2 μg/mL, except Azidothymidine (AZT), which was 3 μg/mL. 10 μL of stock solution was spiked into 190 μL plasma before loading onto the Ostro plate. For blank plasma samples, a 40% ACN solution was used as the spike. For SSLE, a 10 μL stock solution was spiked into 90 μL plasma. The spiked plasma was then diluted with water at a 1:1 ratio before loading onto the SSLE devices.
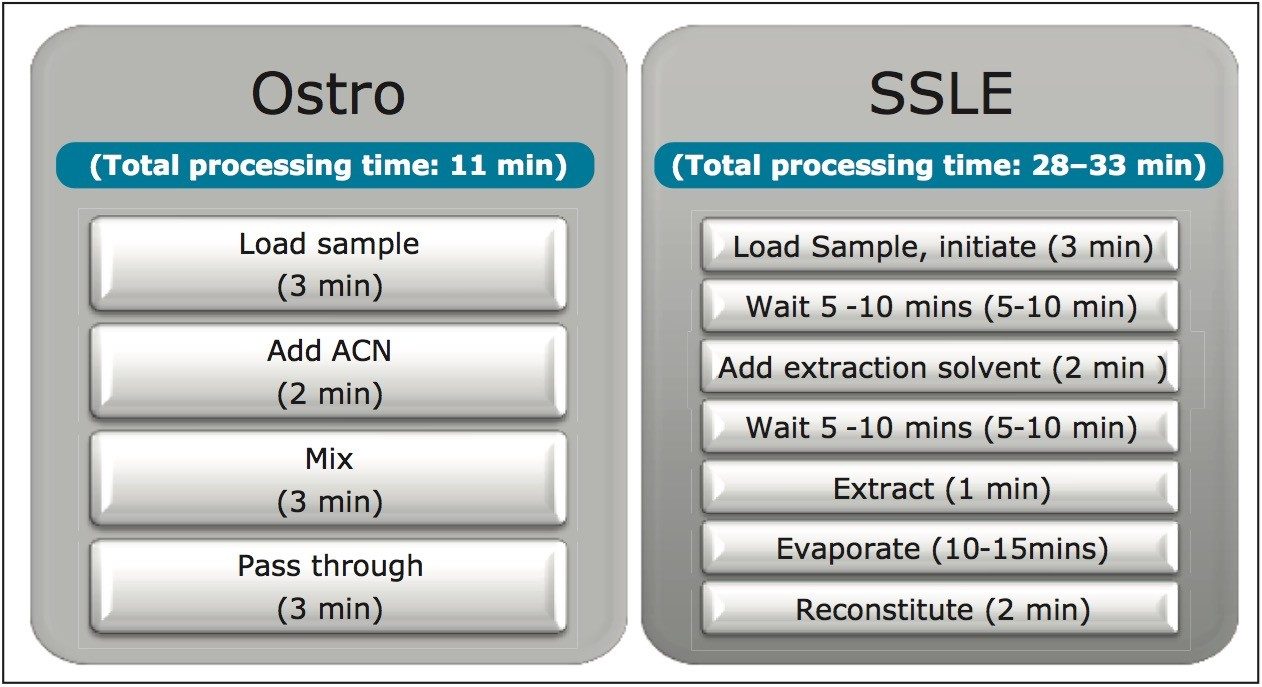
The generic protocol for Ostro Pass-through Sample Preparation was used. 200 μL pre-spiked or blank rat plasma were applied to the plate, then 600 μL of 1% formic acid (FA) in Acetonitrile (ACN) was added followed by manual mixing (to complete protein precipitation). The samples were then eluted using a positive pressure manifold (p/n: 186006961). The eluate is directly injected into the LC-MS system. The Ostro procedure listed below is used independent of the analytes’ chemical properties.
For SSLE, there are multiple dilution buffers (to dilute the biological sample for loading depending on the drug’s functional groups) and extraction solvents (Once an appropriate buffer is selected the method is optimized by screening six different extraction solvents). The protocol we selected for this evaluation is for neutral and basic analytes. 200 μL diluted plasma (100 μL rat plasma + 100 μL water) was loaded into a 200 μL SSLE plate (obtained from a competitor). Loading was initiated by applying vacuum (~3 psi) for 2–5 seconds, waiting 5 minutes for the sample to completely absorb and form the extraction layer. 1 mL of extraction solvent (95:5 (v/v) Dichloromethane: isopropanol (DCM:IPA)) was then applied and allowed to flow for 10 minutes under gravity. Vacuum (10 psi) was applied again for 10–30 seconds to complete the elution. To be compatible with LC-MS analysis, the extract was evaporated to dryness and then reconstituted in 200 μL of 40% acetonitrile (ACN).
Figure 1 shows the protocols and the processing times for both Ostro and SSLE. The total time required to prepare a 96-well plate of plasma samples is 11 minutes for Ostro and at least 30 minutes for SSLE. Ostro is a simple and generic pass through technique that removes both proteins and phospholipids without the need for evaporation and reconstitution, whereas SSLE requires method development with different extraction solvents for different analytes of interest. In addition, the 5–10 minute waiting time after loading is for the loading mixture to contact the sorbent, and the 5–10 minute waiting time after the extraction solvent is applied is for the analytes to interact with the extraction solvent. Since a water-immiscible solvent is used in extraction step, evaporation and reconstitution are required for LC-MS analysis. In addition, the initiation of the flow in the SSLE sample loading step, which is accomplished by applying vacuum (~3 psi) for 2–5 seconds, is very subtle and takes time and practice to perfect. If the initiation time is too short (shorter than 2–5 seconds) or the pressure is too low, the aqueous sample won’t be able to successfully immobilize onto the sorbent. If the time is too long or the pressure is too high, the plasma sample will immediately flow into the collection plate and result in a cloudy elution solution and higher matrix factors. In SSLE, the use of harsh water-immiscible extraction solvents may extract impurities from the frits and plates to contaminate the extraction solution. The extraction solvents such as DCM can also have a negative impact on both operators’ health and the environment long-term.
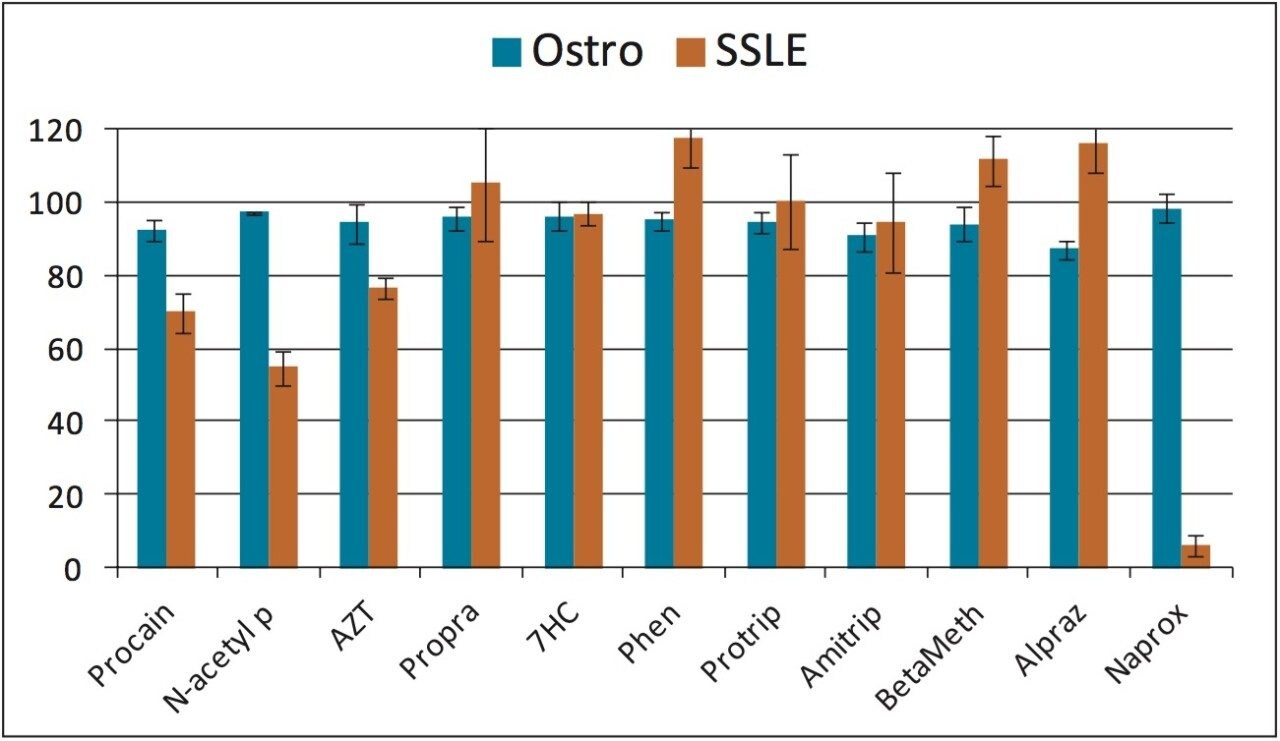
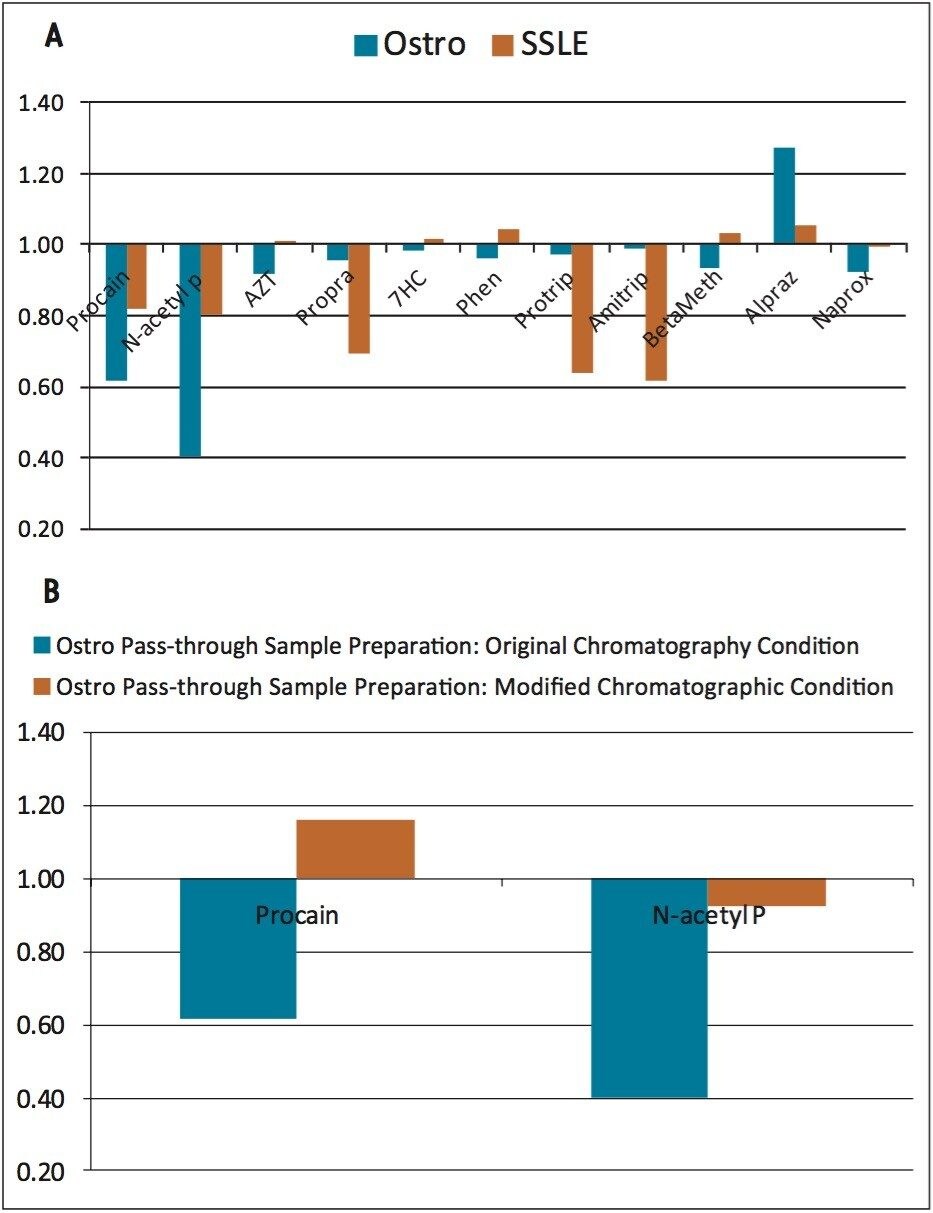
The percent recovery was obtained by the ratio of the peak area of a pre-spiked, extracted sample to the peak area of a post-spiked, extracted sample. With the simple pass-through procedure, Ostro showed very good and consistent recoveries across all the tested analytes (Figure 2) with an average % recovery of 95±4%. For SSLE, as the extraction method was selected for neutral and basic analytes, acidic analytes such as naproxen (“Naprox” in the Figure) were not recovered well at all (less than 10% recovery). In addition, since SSLE is analogous to LLE, in which the extraction is based on the analyte solubility between the aqueous and non-aqueous layers, lower recoveries were observed for polar analytes such as procainamide and N-acetyl procainamide because of the low partition coefficient (Refer to Table 1, “Procain” and “N-acetyl P” in Figure 2, ~60% recovery). Another observation for SSLE is that the analyte recoveries are more variable. The average % RSD for Ostro is 2% and SSLE is 8%, which is presumably due to the initiation step and larger number of overall steps for SSLE compared to Ostro.
The matrix factor was calculated as the ratio of the peak area in the presence of matrix (measured by analyzing blank matrix spiked after extraction with analytes) to the peak area in absence of matrix (elution solvent standard solution of the analytes). The overall matrix factors for Ostro and SSLE are comparable (Figure 3a), where Ostro shows some higher level of interferences for polar analytes such as procainamide and N-acetyl procainamide, and SSLE shows higher matrix factors for propranolol, protriptyline and amitriptyline. The higher matrix factors for polar analytes on Ostro may be caused by co-eluting salts in the beginning of the gradient since Ostro is a pass through sample preparation technique. This can be improved by a simple change in chromatographic conditions (results shown in Figure 3b). In this case, instead of starting at 20% mobile phase B, the gradient was changed to start at 5% mobile phase B. In the later case, the polar analytes do not co-elute with the hydrophilic interferences (suspected salts). As expected, the matrix factors for these analytes (procainamide and N-acetyl procainamide) were reduced to 1±0.15 as opposed to 1±0.5 before the change in the initial gradient conditions.
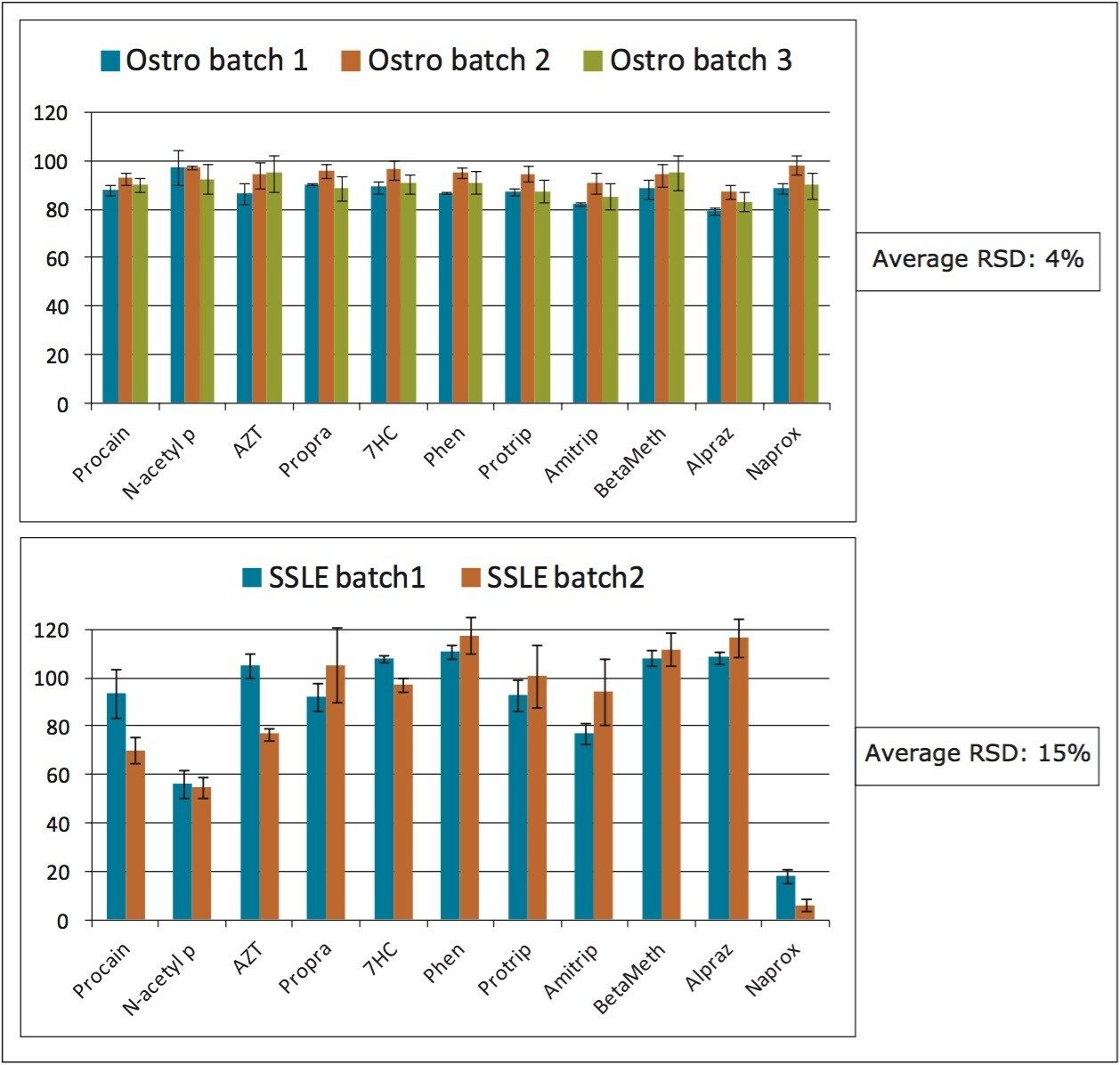
A critical attribute of any sample preparation in bioanalysis is the ability to obtain the same results over a long period of time (life of drug). Therefore, it is important to evaluate the batch-to-batch reproducibility of any sample preparation product. To compare the long term viability of Ostro and SSLE, analyte recoveries for 3 different batches of Ostro and 2 different batches of SSLE were performed (see Figures 4a and 4b). Ostro showed very consistent batch-to-batch recovery for the analytes tested, with an average % RSD in recovery of 4%. Recovery and reproducibility for SSLE was less consistent, with % deviation for average recovery being 15%. These data show that the results with a simple, pass through sample preparation protocol give much better reproducibility needed for a bioanalytical assay that spans the life of a drug, with the additional advantage of saving 60% of the processing time compared to SSLE.
Ostro is a pass-through sample preparation technique that has several advantages over SSLE, including:
720005199, October 2014