Improved Recovery of a Lipid Conjugated Antisense Oligonucleotide from Human Plasma using the OligoWorks™ SPE Microplate Kit and an Optimized Protocol
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
Developing bioanalytical sample preparation and LC-MS methods for oligonucleotides in ADME/DMPK research is challenging due to their structural diversity, low circulating concentrations, complex biological matrices (e.g., plasma, urine, tissues), and strong binding to endogenous proteins, which leads to low extraction recoveries and reduced method sensitivity. An additional challenge arises from the need to use small sample volumes, as large molecule therapies generally have lower MS sensitivity compared to small molecules. The variety of extraction techniques further complicates method development, requiring significant time and expertise. This challenge intensifies when oligonucleotide therapeutics are modified with residues and conjugates, that can further impair their recovery. Therefore, a simple, kit-based approach is needed that can handle different biomatrices and sample volumes, can be easily optimized, and can be implemented by scientists with limited experience in the workflow.
Introduction
Oligonucleotide therapies have grown rapidly due to the rising demand for gene therapy and precision medicine. As of July 2024, there are ≥21 oligonucleotide-based therapies approved in the US, with ≥130 in clinical development for a variety of diseases, including genetic, cardiovascular, neurological, and cancer.1&2 This growth has increased the need for highly sensitive and accurate LC-MS quantification from biological samples in support of their research and development. However, challenges remain, including assay sensitivity, small sample volumes, and complex sample preparation, especially for modified oligonucleotides with conjugated moieties like lipids.3 There is a need for simpler, standardized sample preparation and extraction workflows capable of achieving high extraction efficiency to facilitate low ng/mL sensitivity from low sample volumes of ≤100 µL.
Results and Discussion
The Solution
OligoWorks SPE Kits are versatile, broadly applicable, and automation-friendly sample preparation tools, which provide premeasured, lot-traceable reagents optimized for precise and reliable LC-MS quantification of oligonucleotide therapeutics from biological matrices such as plasma, urine, and organ tissue. Key features of the kit include: protocol designed for efficient retention and elution of a variety of oligonucleotides, highly efficient sample Proteinase K pretreatment to thoroughly disrupt oligonucleotide to biomatrix protein binding, a mixed-mode WAX SPE purification in the microplate format for enhanced recovery and selectivity, and sample concentration with an MS compatible eluate, which eliminates the need for sample evaporation and reconstitution prior to LC-MS analysis.
The OligoWorks WAX sorbent, is a polymeric reversed-phase, weak anion exchange mixed-mode material, selected for its anion exchange binding capacity and selectivity for the negatively charged backbones of oligonucleotides. Compared a traditional reversed-phase material, this OligoWorks SPE WAX sorbent is effective at retaining oligonucleotides without needing to employ an ion pairing agent. Additionally, this sorbent is QC-tested and batch-selected to ensure optimal oligonucleotide recovery.4 The 96-well Microplate SPE device in the µElution format allows for elution in as little as 25 µL, enabling significant concentration factors of sample extracts without the need to evaporate and reconstitute the extract prior to LC-MS analysis. An entire OligoWorks SPE Microplate can be processed in under 20 minutes using either vacuum or positive pressure, and can be easily automated for greater throughput and ‘walk-away’ time for the scientist, improving laboratory efficiency.
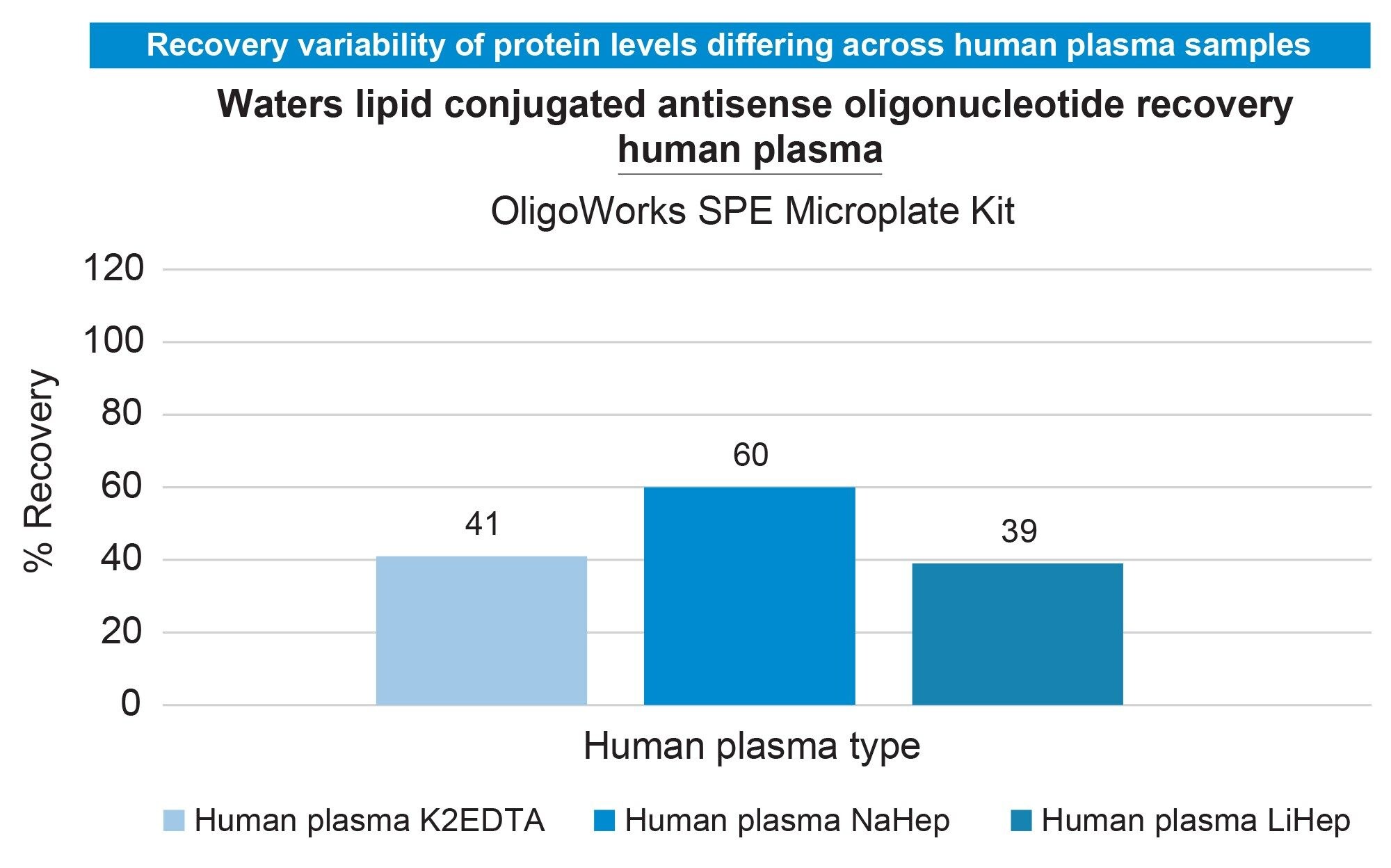
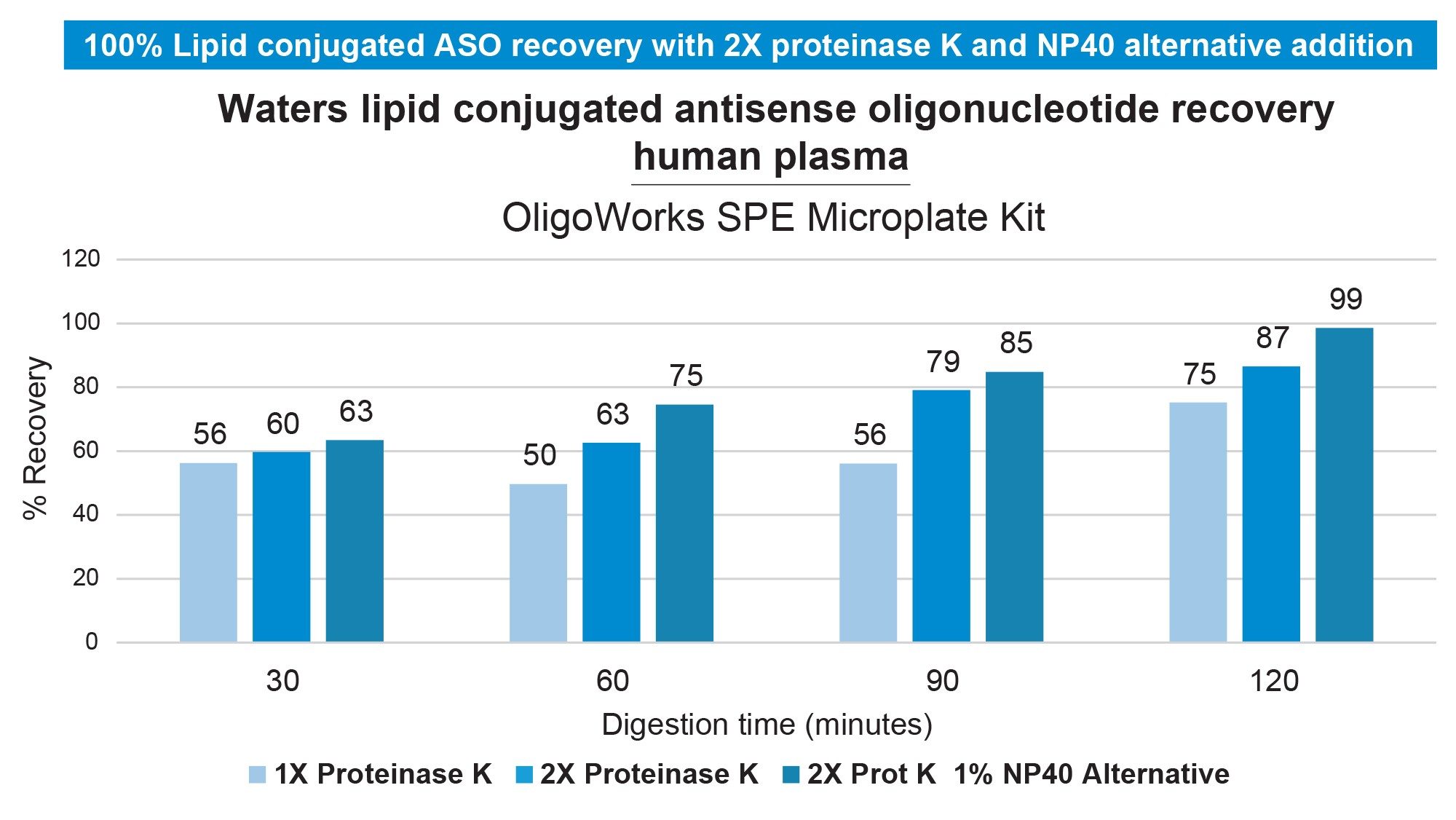
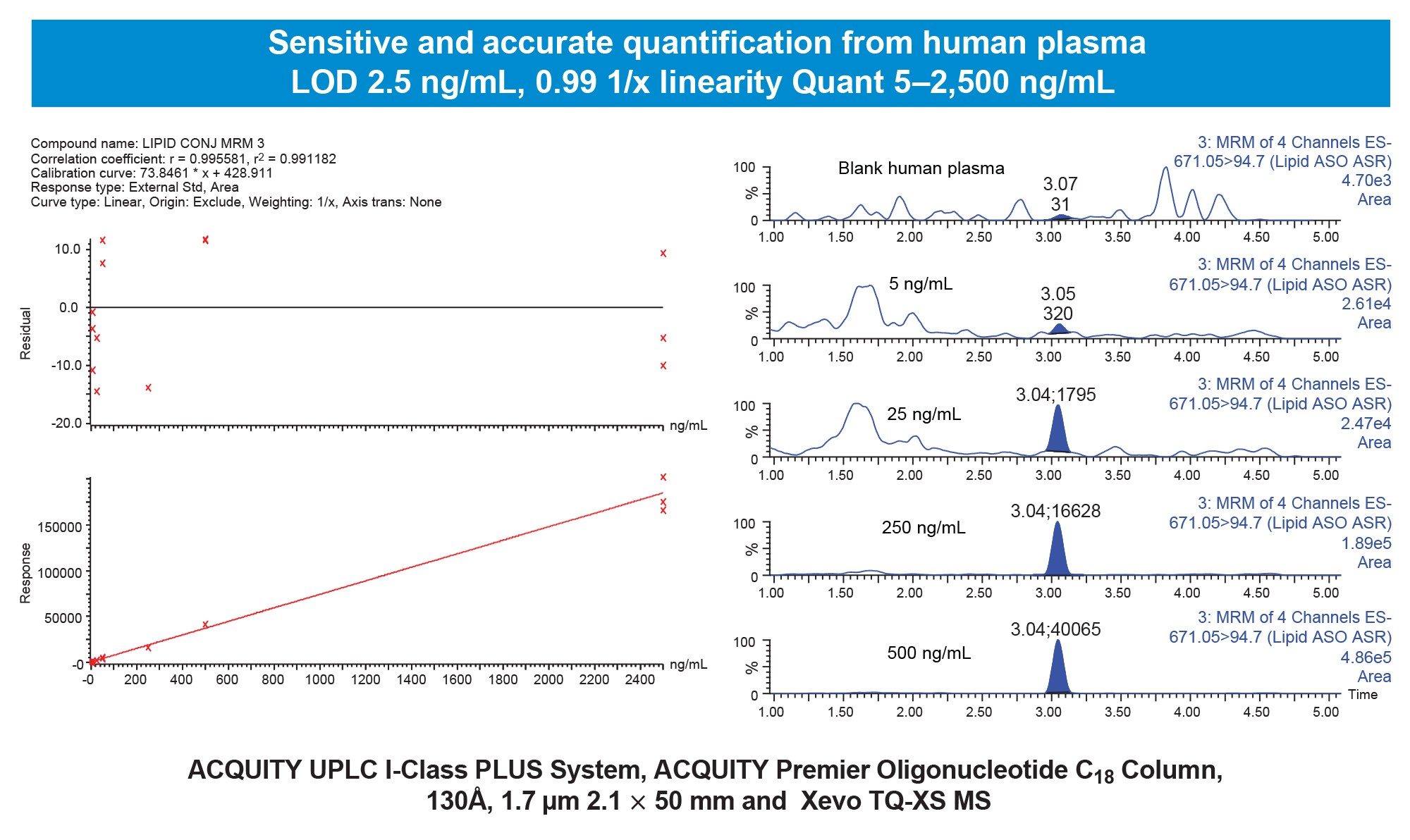
Preliminary experiments evaluated the human plasma recovery of the Waters Lipid Conjugated ASO LC-MS standard (16-mer ASO with palmitate, phosphorothioated backbone, and methoxy ethyl modifications, Waters p/n: 186010747) using the OligoWorks SPE Microplate Kit and its standard protocol (Figure 1). In these tests, 100 µL of plasma (with K2EDTA, NaHep, or LiHep anticoagulants) was spiked with the Lipid Conjugated ASO (0.1 pmol/µL). The OligoWorks RapiZyme Proteinase K Digestion Module reagents (p/n: 186010601) were added, vortexed, and incubated at 55 °C for 1 hour. After proteinase K digestion, the plasma samples (180 µL) were purified using WAX SPE with the OligoWorks SPE Microplate. Low to moderate recovery was observed across plasma pools (Figure 2). By adjusting the plasma pretreatment—extending digestion time, doubling proteinase K, adding NP-40 Alternative (1%), and subsequent purification using the OligoWorks SPE Microplate, we achieved full recovery from human plasma (99%; Figure 3). Figure 4 demonstrates accurate and sensitive quantification of the lipid-conjugated ASO from human plasma (NaHep), with ≥0.99 linearity, a dynamic range of 5-2,500 ng/mL, and accuracy within ±15% at all calibration points, using the optimized OligoWorks SPE Microplate Kit protocol.
Note: LC-MS/MS multiple reaction monitoring (MRM) analysis was performed using a Waters Xevo™ TQ-XS Tandem Quadrupole Mass Spectrometer using negative electrospray ionization (ESI-)and a chromatographic separation using an ACQUITY™ I-Class PLUS UPLC™ System and ACQUITY Premier Oligonucleotide BEH™ C18, 1.7 µm, 2.1 x 50 mm Column (Waters, p/n: 186009484). An ACQUITY Premier LC System with Xevo TQ Absolute Mass Spectrometer can be applied in this workflow to further improve sensitivity, precision, and accuracy of the separation.
Conclusion
A standardized, kit-based approach (with a reliable starting protocol, pre-measured, QC-verified, and lot-traceable reagents) significantly reduced sample method development time and enabled easy protocol optimization. Modifying the sample pretreatment protocol-extending digestion time, doubling the proteinase K amount, and employing a low concentration of the nonionic surfactant NP-40 Alternative yielded full recovery of the lipid-conjugated ASO from plasma. Using this optimized kit-based approach, lower limits of quantification of 5 ng/mL were achieved with just 100 µL of human plasma.

OligoWorks, RapiZyme, QuanRecovery, MaxPeak, BEH, ACQUITY, UPLC and Xevo are trademarks of Waters Technologies Corporation. All other trademarks are the property of their respective owners.
References
- “Oligonucleotides Market Outlook 2034: Projected to Reach US$ 13.1 Billion by 2034, Driven by Expanding Applications in Biotechnology and Precision Medicine” (December 02, 2024) Source:Transparency Market Research. https://www.globenewswire.com/news-release/2024/12/02/2989923/0/en/Oligonucleotides-Market-Outlook-2034-Projected-to-Reach-US-13-1-Billion-by-2034-Driven-by-Expanding-Applications-in-Biotechnology-and-Precision-Medicine-TMR.html
- “Overview and Outlook for RNA-Based Therapies” (May 2024). Source:Lilly. Whitepaper. (Lit Code 20240522-Lilly-RNA-Based-Therapies-White-Paper-vFINAL) https://avalere.com/wp-content/uploads/2024/06/20240522-Lilly-RNA-Based-Therapies-White-Paper-vFINAL.pdf
- Tran P, Weldemichael T, Liu Z, Li HY. Delivery of Oligonucleotides: Efficiency with Lipid Conjugation and Clinical Outcome. Pharmaceutics. 2022 Feb 1;14(2):342. doi: 10.3390/pharmaceutics14020342. PMID: 35214074; PMCID: PMC8879684.)
- Makda Araya, Abraham S. Finny, Margot Lee, Mary Trudeau, Nicole Lawrence, Frank J Marszalkowski Jr., Jessica Field, Balasubrahmanyam Addepalli, Matthew A. Lauber. Elements of Robust SPE-Based Oligonucleotide Extraction, Waters Application note, 720008414, 2023.
720008676, February 2025