In this application note we present the utility of RapiFluor-MS coupled with UPLC-FLR-MS for monitoring labeled glycans ranging across a range of properties, masses, and relative abundance.
Glycosylation of is a complex and critical aspect of most therapeutic proteins which must be well characterized. Often, the profile of N-glycans is identified as a critical quality attribute and as a result is monitored throughout the lifecycle of products. As discussed in this application note, preparation of samples with a GlycoWorks RapiFluor-MS N-Glycan Kit can dramatically reduce sample preparation time and complexity.
In addition, the use of RapiFluor-MS yields improved FLR sensitivity and dramatically improved MS sensitivity. Through improving glycan MS sensitivity RapiFluor-MS labeling permits the use of mass detection with the ACQUITY QDa and thereby affords greater confidence in peak monitoring across the range of structures encountered during biopharmaceutical development.
Used together, RapiFluor-MS labeling and HILIC-FLR-MS with ACQUITY UPLC H-Class Bio System and the ACQUITY QDa Mass Detector offer an unparalleled solution for monitoring the N-glycan profiles of biotherapeutics.
Glycosylation is one of the most complex post-translational modifications of protein-based biotherapeutics. The efficacy of glycosylated therapeutics is directly related to the glycoprofile. The presence of undesired structures can lead to changes in PK/PD profiles, either positively or negatively, and have been associated with immunogenic responses. For these reasons glycosylation is often designated as a critical quality attribute (CQA). During the development process, the glycoprofile of candidate molecules is extensively studied and characterized. Characteristic profiles are then monitored through process development, commercialization, and post-approval studies to maintain product efficacy and safety.
In this application note, we present a streamlined approach to label released N-glycans with RapiFluor-MS and analyze the labeled N-glycans with the ACQUITY UPLC H-Class Bio System with fluorescent (FLR) and ACQUITY QDa Mass Detectors. This new monitoring workflow allows researchers to prepare samples from glycoprotein to UPLC-FLR/MS analysis in 30 minutes. In addition to reduced sample preparation times, RapiFluor-MS yields 14 times greater fluorescence response and 160 times greater MS response when compared to 2-AB. These improvements enable the use of FLR and mass detection with the ACQUITY QDa for routine analysis. In this application note we present the utility of RapiFluor-MS coupled with UPLC-FLR-MS for monitoring labeled glycans ranging across a range of properties, masses, and relative abundance.
|
LC System: |
ACQUITY UPLC H-Class Bio |
|
Detectors: |
ACQUITY UPLC FLR and ACQUITY QDa Mass Detector |
|
Column: |
ACQUITY UPLC Glycan BEH Amide, 130Å, 1.7 μm, 2.1 x 150 mm |
|
Column temp.: |
60 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
2 μL |
|
Data management: |
Empower 3 FR2 CDS |
|
Data rate: |
5 points/sec. |
|
Excitation wavelength: |
265 nm |
|
Emission wavelength: |
425 nm |
|
Sample rate: |
5 points/sec |
|
Mass range: |
500–1250 Da |
|
Cone voltage: |
15 V |
|
Capillary voltage: |
1.5 kV |
|
Probe temp.: |
500 °C |
|
Ionization mode: |
ESI+ |
|
Mobile phase A: |
Acetonitrile (Pierce, LC-MS Grade) |
|
Mobile phase B: |
50 mM ammonium formate, pH 4.4, (LC-MS Grade, Waters ammonium formate concentrate) |
|
Mobile phase C: |
Acetonitrile (LC-MS grade) |
|
Mobile phase D: |
Acetonitrile (LC-MS grade) |
|
Time |
Flow rate(mL/min) |
%A |
%B |
%C |
%D |
|---|---|---|---|---|---|
|
Initial |
0.4 |
75 |
25 |
0 |
0 |
|
35 |
0.4 |
54 |
46 |
0 |
0 |
|
36.5 |
0.2 |
0 |
100 |
0 |
0 |
|
39.5 |
0.2 |
0 |
100 |
0 |
0 |
|
42.5 |
0.2 |
75 |
25 |
0 |
0 |
|
47.4 |
0.4 |
75 |
25 |
0 |
0 |
|
55 |
0.4 |
75 |
25 |
0 |
0 |
A sample of murine IgG1 mAb N-Glycans was prepared from Waters Intact mAb Mass Check Standard (p/n 186006552), which is included in the GlycoWorks RapiFluor-MS N-Glycan Kit (p/n 176003606). N-Glycans were also prepared from RNase B and bovine fetuin (Sigma Aldrich). Released and labeled N-glycan pools were generated using the GlycoWorks RapiFluor-MS N-Glycan Kit following the protocol provided in the Care and Use Manual (715004793). Following release and labeling, samples were dried using a CentriVap and reconstituted in 25 µl of a mixture of ACN/Water/DMF at a ratio of 22.5%:55.5%:22%, respectively. In each case the targeted mass load was 30 pmoles of released glycan. The ammonium formate mobile phase was prepared using Waters ammonium formate concentrate (p/n 186007081).
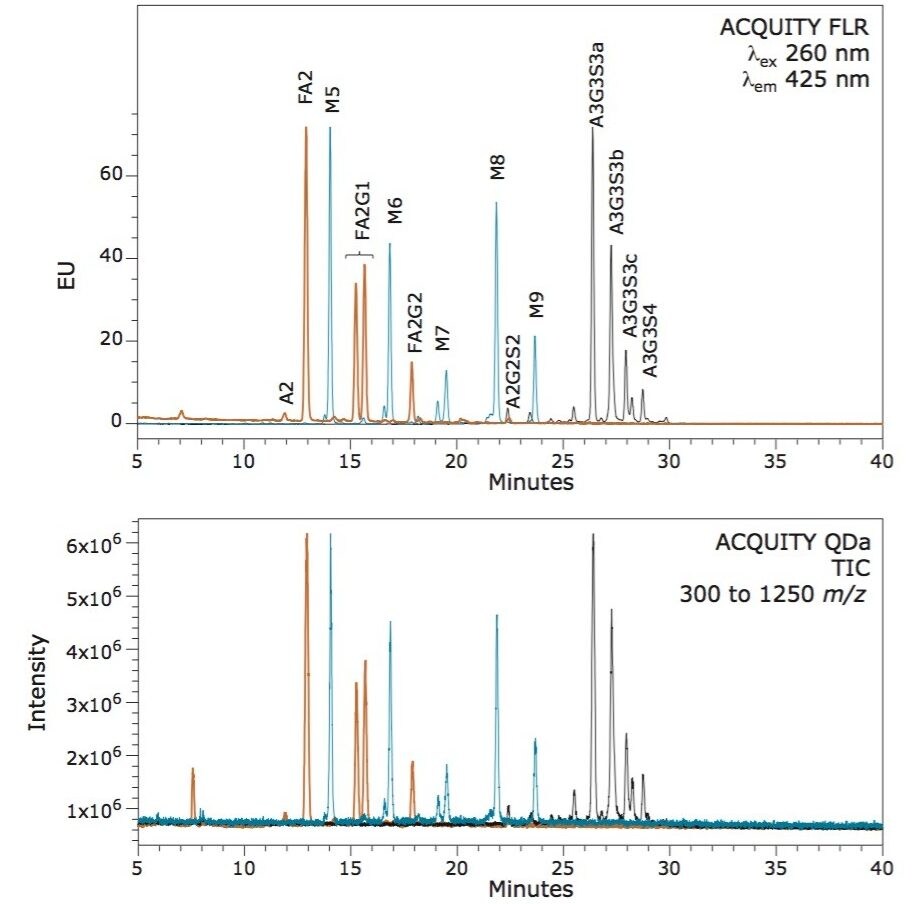
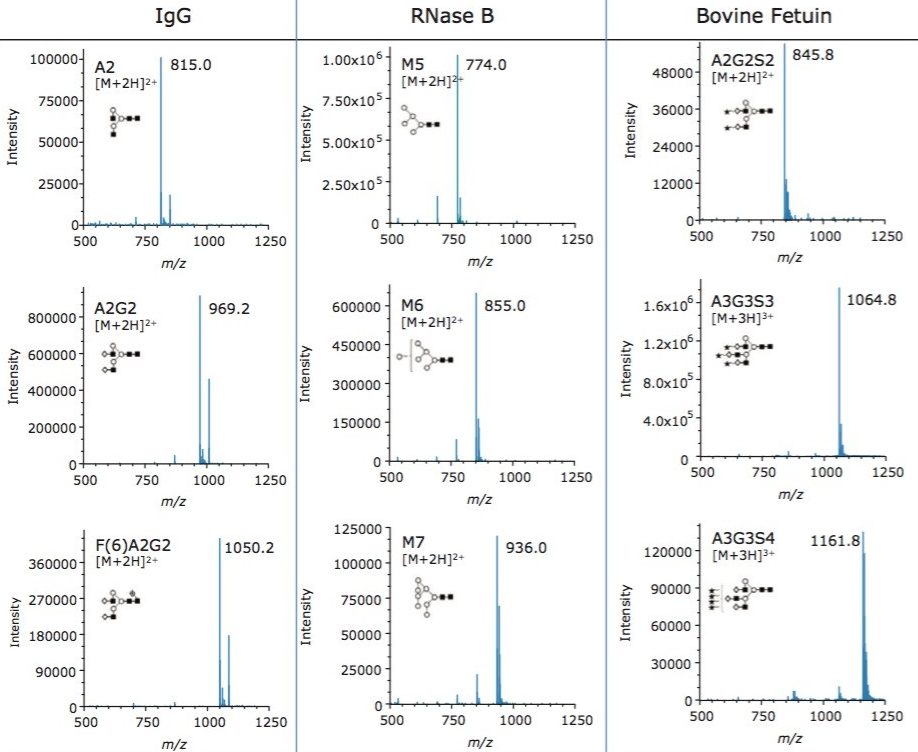
N-glycosylation is a non-template driven process that generates a vast array of glycan structures that vary in size, charge, and extent of branching depending on the protein and expression system. To evaluate the capacity of the ACQUITY QDa to detect glycans both within and beyond its mass range, three glycoproteins (human IgG, RNAse B, and bovine fetuin) were selected to provide typically observed glycans ranging from neutral bi-antennary structures to tetra-sialylated structures. N-glycans from each protein were released using Rapid PNGase F and labeled with RapiFluor-MS following the provided sample preparation protocol. Labeled glycans were separated via UPLC-HILIC and detected using both an ACQUITY FLR and ACQUITY QDa.
As is evident in Figure 1, each glycan structure is chromatographically resolved using a single gradient method. In addition, each glycan structure observed in fluorescence (top panel) is also observed by the ACQUITY QDa Mass Detector (bottom panel), indicating the ability of the ACQUITY QDa to detect glycans across a range of possible structures and attributes when labeled with RapiFluor-MS. For traditional labeling technologies this is not possible due to poor ionization efficiency.
While it is useful that glycan structures can be observed by mass detection, it is important to understand the quality of the resulting spectra and the charge states of the glycan ions obtained within. To understand this aspect, we integrated peaks spanning a range of glycan properties and measured the relative abundances of species in each sample using FLR integrated data. The spectra shown in Figure 2 demonstrate the ability of the ACQUITY QDa to generate high quality spectra for glycan structures across a wide range of properties and masses. The data also demonstrate that both high and low abundance glycan structures can be readily detected. Our data indicates that high quality spectra are generated for structures present in the fluorescence profiles at abundances as low as 0.5% highlighting the sensitivity of ACQUITY QDa mass detection combined with the improved ionization efficiency afforded by RapiFluor-MS. Our data also demonstrate how the improved charging of glycan structures by the use of RapiFluor-MS allows small structures such as A2, as well as very large structures, such as the tetrasialylated A3G3S4, to be detected with the QDa.
Glycosylation of is a complex and critical aspect of most therapeutic proteins which must be well characterized. Often, the profile of N-glycans is identified as a critical quality attribute and as a result is monitored throughout the lifecycle of products. As discussed in this application note, preparation of samples with a GlycoWorks RapiFluor-MS N-Glycan Kit can dramatically reduce sample preparation time and complexity. In addition, the use of RapiFluor-MS yields improved FLR sensitivity and dramatically improved MS sensitivity. Through improving glycan MS sensitivity RapiFluor-MS labeling permits the use of mass detection with the ACQUITY QDa and thereby affords greater confidence in peak monitoring across the range of structures encountered during biopharmaceutical development. Taken together, RapiFluor-MS labeling and HILIC-FLR-MS with ACQUITY UPLC H-Class Bio System and the ACQUITY QDa Mass Detector offer an unparalleled solution for monitoring the N-glycan profiles of biotherapeutics.
720005353, March 2015