Supercritical fluid chromatography (SFC) has become a mainstream technology for chiral separations based on its higher efficiency, throughput, and wide applicability. For purification purposes, chiral SFC has seen increased interest and applicability, becoming the technique of choice in many cases. Purification of chiral compounds by SFC typically utilizes isocratic method conditions and stacked injections for optimal collection efficiency. In this application note, bucetin will be used as an example compound to demonstrate the typical work flow for chiral purification by SFC. The process will include gradient screening and isocratic method development on the ACQUITY UPC2 System, scale-up and purification using stacked injections on the Prep 80q SFC System, and post purification analysis on the ACQUITY UPC2 System.
Generally, when purification is the goal, it is more practical for method development to be performed at the analytical scale to save solvent, time and sample. Once the method has been optimized at the analytical scale, calculations based on column size and system volume are utilized to scale-up the separation for purification. For successful scale-up, identical sample concentration, diluents, mobile phase and average system pressure (specifically for SFC) are required. The analytical and preparative column chemistries must be identical and it is beneficial to maintain particle size and column length.1
Supercritical fluid chromatography (SFC) has become a mainstream technology for chiral separations based on its higher efficiency, throughput, and wide applicability. For purification purposes, chiral SFC has seen increased interest and applicability, becoming the technique of choice in many cases.2 Purification of chiral compounds by SFC typically utilizes isocratic method conditions and stacked injections for optimal collection efficiency. In this application note, bucetin will be used as an example compound to demonstrate the typical work flow for chiral purification by SFC. The process will include gradient screening and isocratic method development on the ACQUITY UPC2 System, scale-up and purification using stacked injections on the Prep 80q SFC System, and post purification analysis on the ACQUITY UPC2 System.
The racemic bucetin sample was made up at a concentration of 10 mg/mL in HPLC grade methanol.
Analytical chromatography, method development and supporting fraction analysis were performed on the ACQUITY UPC2 System with a PDA Detector and controlled by MassLynx Software. Scale-up and purification was done on the Prep 80q SFC System with a 2489 UV/Vis Detector and ChromScope Software control.
General running conditions for the ACQUITY UPC2 and the Prep 80q SFC System are listed below. All other conditions will be noted in the figures.
|
ACQUITY UPC2 analytical method conditions |
|
|---|---|
|
Control: |
MassLynx Software |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Methanol (MS Grade) |
|
Flow rate: |
3 mL/min |
|
Pressure: |
120 bar |
|
Temperature: |
35 °C |
|
PDA detector: Scan: |
220 – 400 nm Extracted wavelength: 247 nm |
|
Analytical column: |
Chiralpak IA Column (4.6 x 150 mm, 5 μm) |
|
Prep 80q SFC preparative method conditions |
|
|---|---|
|
Control: |
ChromScope Software |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Methanol (HPLC grade) |
|
Flow rate: |
62 mL/min |
|
Pressure: |
120 bar |
|
Temperature: |
40 °C |
|
UV/Vis detector: |
247 nm |
|
Preparative column: |
Chiralpak IA Column (21 x 150 mm, 5 μm) |
Method development in chiral SFC involves a column screening step using a gradient, followed by developing an isocratic method for purification on the selected column chemistry. Optimization is done to determine isocratic conditions that allow high loading and short cycle times. To determine a starting point for isocratic method development, the retention time and slope of the gradient are used to calculate the %B when the peak eluted. Typically, for better resolution and higher loading the calculated %B is adjusted 10% lower.
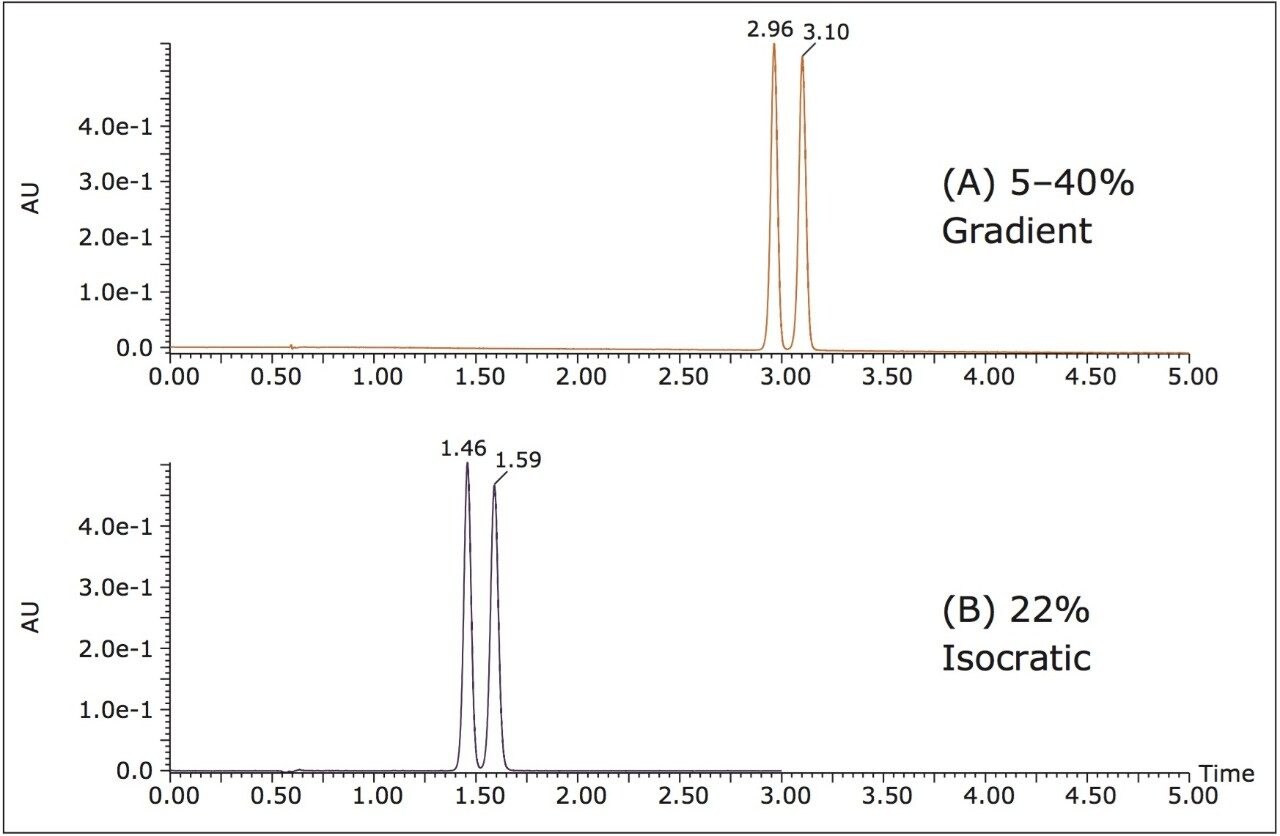
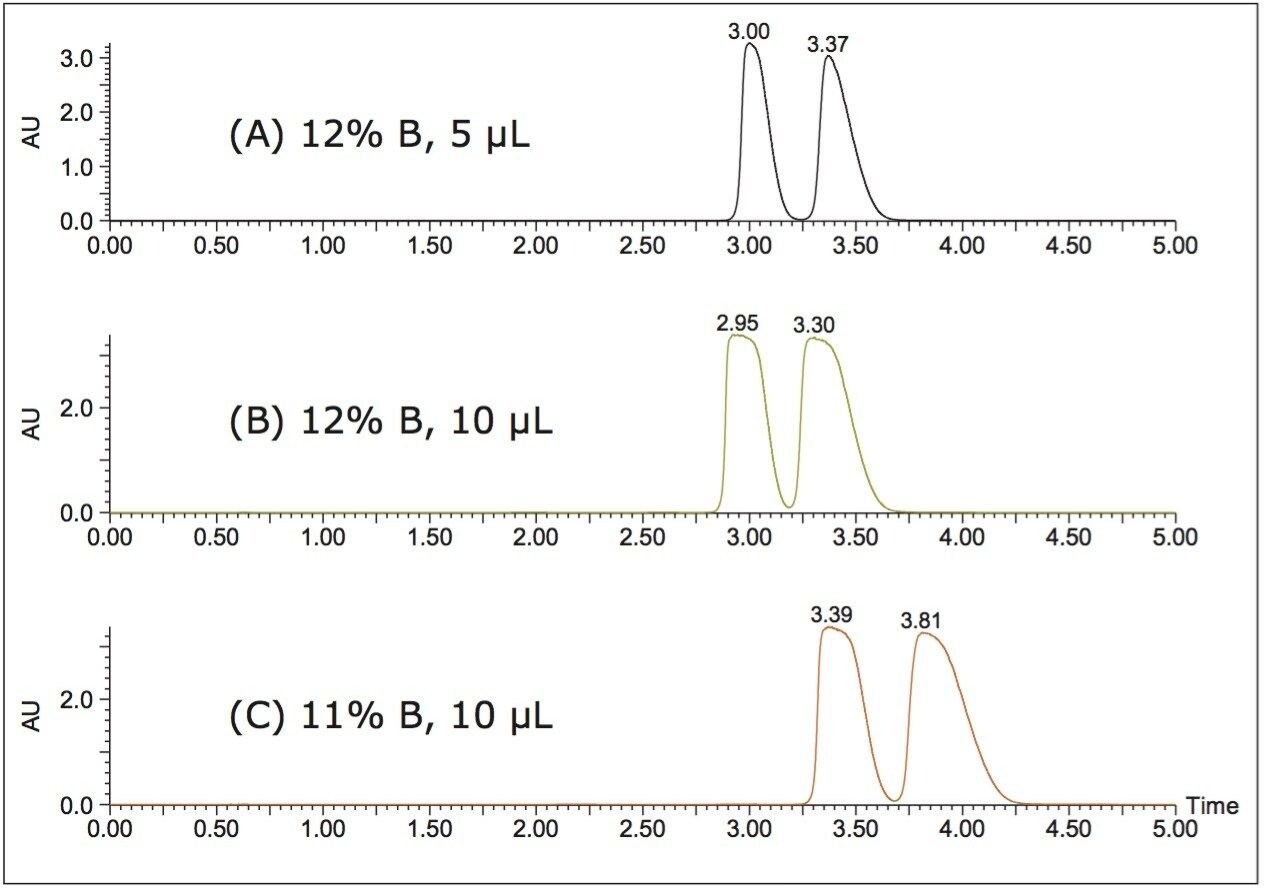
In this case, the gradient conditions were 5–40% methanol in 5 minutes. At those conditions the average retention time of the two bucetin peaks was 3.03 min. After adjusting for a 0.6 min offset due to system volume, the %B at the elution time was calculated to be 22% methanol. Figure 1 shows chromatograms of (A) the bucetin separation under the gradient conditions, and (B) the separation under 22% methanol isocratic conditions. The two methods show very similar resolution for relatively low loading (2 μL), but not enough resolution for purification purposes. To improve loading, a 12% isocratic method was investigated (10% lower than the calculated percentage), the resulting chromatograms are in Figure 2 (A) and (B). Higher loading was achieved and the resolution was acceptable, however to improve the 10 μL separation even further a final 11% isocratic method was used (Figure 2 (C)).
Proper scaling ensures that the chromatographic profile of a sample at the preparative scale will be identical to the chromatography obtained at the analytical scale.3 To maintain separation quality, the columns were identical in chemistry, particle size and length, and the system pressure was matched between the two systems. Geometric scaling of the flow rate and injection volume was performed based on the inner diameters (I.D.s) of the analytical and preparative columns using the Waters Prep Calculator. The flow rate of 3 mL/min and injection volume of 10 μL on the 4.6 mm analytical column equated to a flow rate of 62 mL/min and injection volume of 200 μL on the 21 mm prep column.
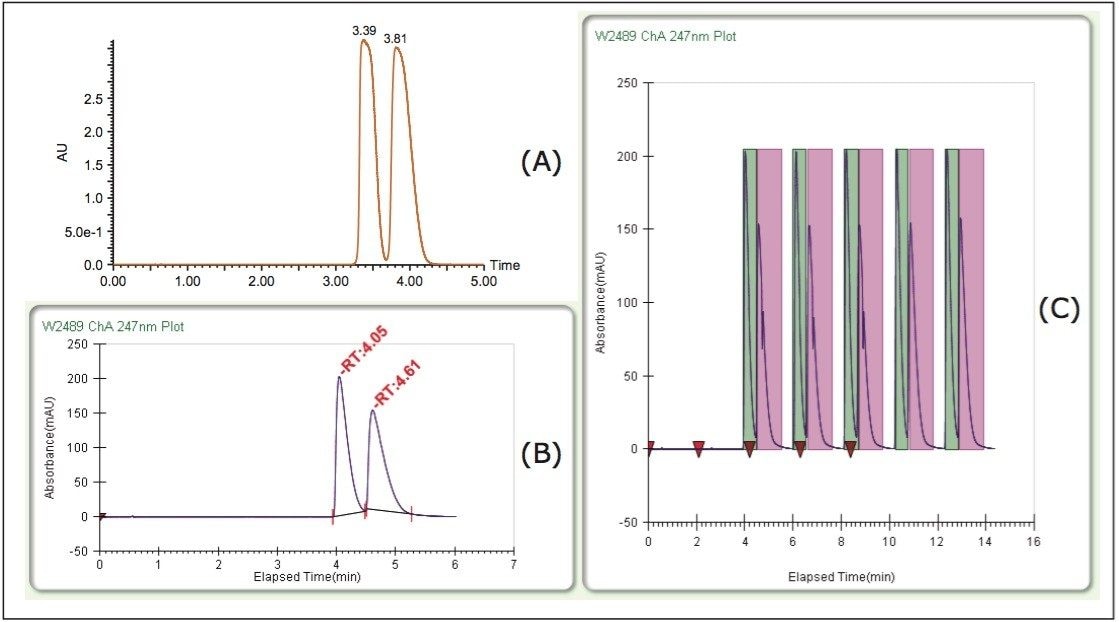
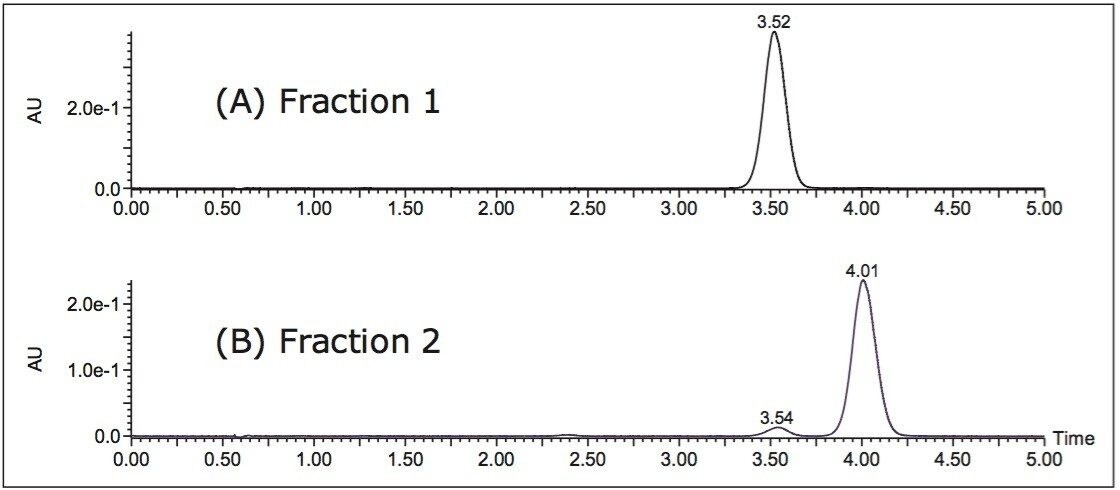
In Figure 3, the ACQUITY UPC2 (A) and scaled-up Prep 80q SFC System (B) chromatograms are shown along with the final purification utilizing stacked injections and timed collections (C). The use of stacked injections along with 200 μL loading allowed for 10 mg of sample to be processed in about 14 minutes. The first three sets of peaks show injection spikes from the injections made during the run, when no injections were made (sets 4 and 5) no injection spike is observed. Given that the initial sample was a racemic mixture, each enantiomer started out at a purity of 50%. The fractions obtained on the Prep 80q SFC System, were analyzed on the ACQUITY UPC2 System (Figure 4), and the results revealed purities of 100% for peak 1 and 95% for peak 2.
720005475, August 2015