In this application note we describe a highly specific and sensitive methodology for the quantification of desmopressin in plasma using solid phase extraction (SPE) followed by reversed-phase UPLC coupled to tandem quadrupole mass spectrometry.
The low circulating levels of desmopressin requires a high-sensitivity assay to accurately define its pharmacokinetics in humans, especially pediatrics. The combination of solid-phase extraction methodology, UPLC chromatography, and Xevo TQ-S mass spectrometer has facilitated the development of an assay for desmopressin with a LLOQ of 1 pg/mL in plasma.
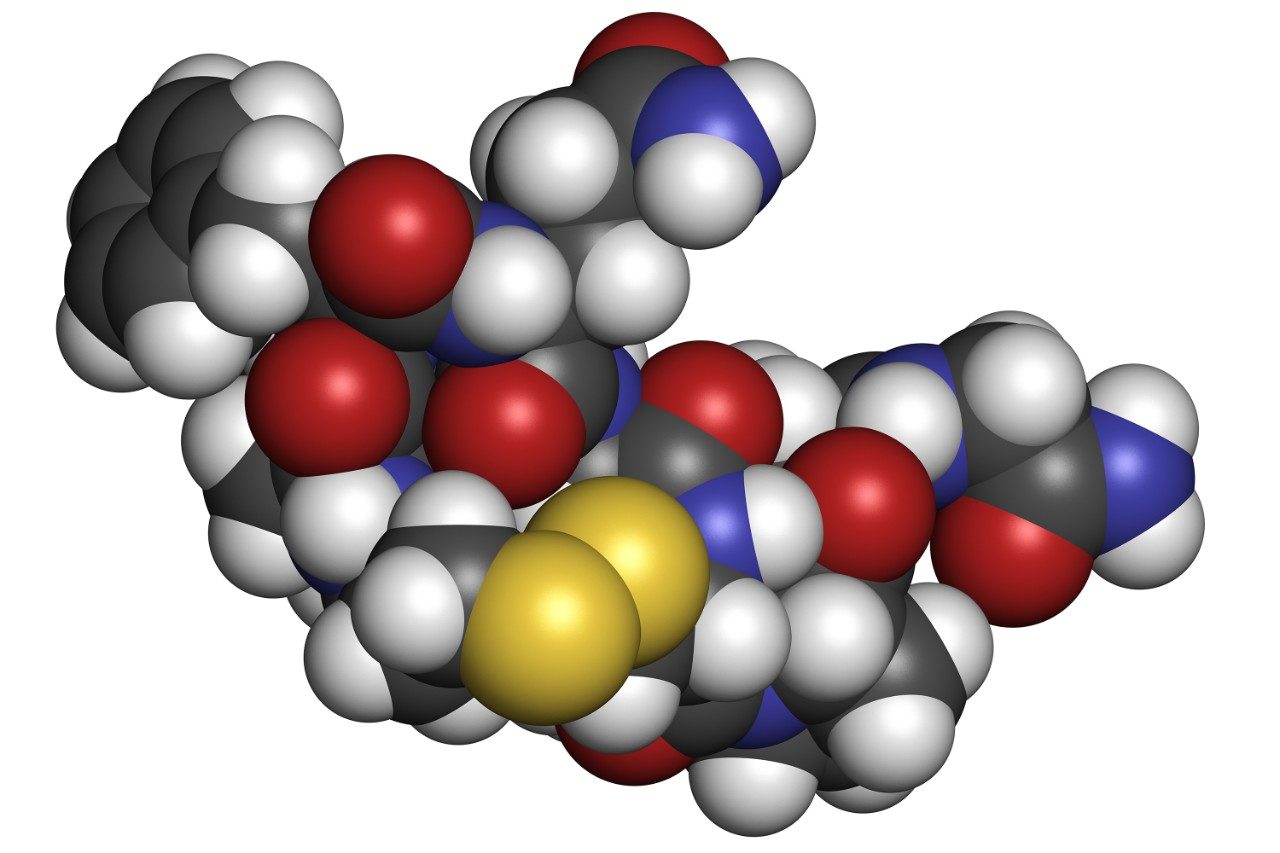
Desmopressin (1-desamino-8-D-arginine vasopressin), Figure 1, (trade names: DDAVP, Stimate, Minirin) is a peptide containing nine amino acids, a form of the normal human hormone arginine vasopressin that reduces urine production. It may be taken nasally, intravenously, or as a tablet. Desmopressin is most commonly prescribed for the treatment of diabetes insipidus or nocturnal enuresis.
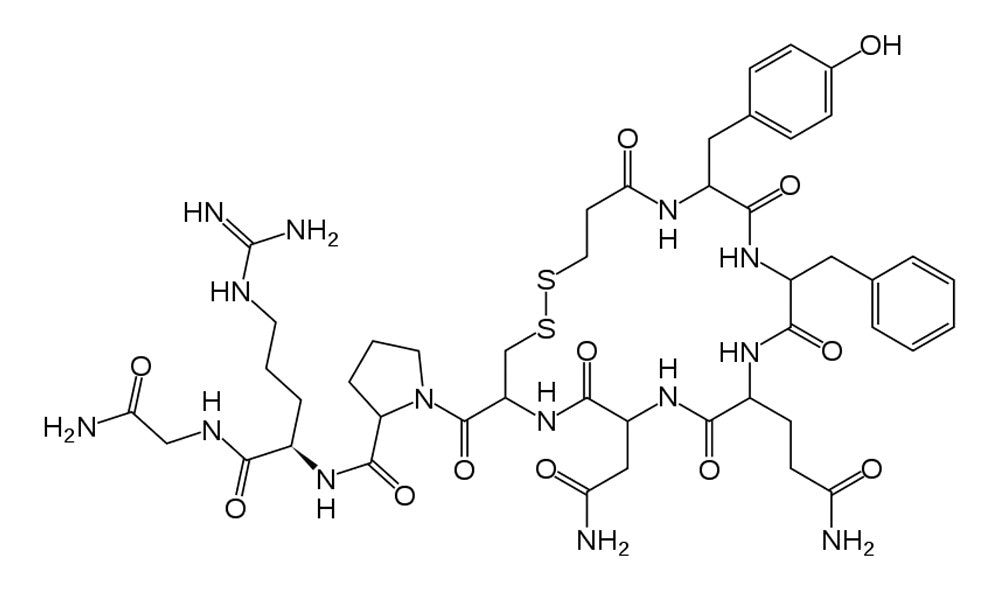
Compared to vasopressin, desmopressin’s first amino acid has been deaminated, and the arginine at the eighth position is in the dextro rather than the levo form. Desmopressin works by limiting the amount of water that is eliminated in the urine. Desmopressin binds to V2 receptors in renal collecting ducts, increasing water reabsorption. It also stimulates release of von Willebrand factor from endothelial cells due to stimulation of the V1a receptor.
Desmopressin is degraded more slowly than recombinant vasopressin, and requires less frequent administration. In addition, it has little effect on blood pressure, while vasopressin may cause arterial hypertension.
Desmopressin is typically dosed at level of 0.3 mcg DDAVP/kg body weight and is mainly excreted in the urine with a terminal half-life from three hours in normal healthy patients to nine hours in patients with severe renal impairment. The peak plasma concentration levels are observed at one to one-and-a-half hours after dosing by nasal administration. This dosing level and rapid clearance results in a circulating level in the low pg/mL range.
In order to accurately determine the pharmacokinetics of desmospressin, it is necessary to have an assay with a limit of detection at least 20 to 100 times lower than the Cmax value, in this case on the order of 1 to 2 pg/mL. As this compound is an analogue of a naturally occurring peptide, the development of a high-sensitivity, robust, and reliable assay requires selective sample isolation and separation from the endogenous materials in blood products as well as a highly specific and sensitive mechanism of detection.
In this application note we describe a highly specific and sensitive methodology for the quantification of desmopressin in plasma using solid phase extraction (SPE) followed by reversed-phase UPLC coupled to tandem quadrupole mass spectrometry.
The samples were isolated using SPE employing a Waters Oasis WCX cartridge. A 500 μL aliquot of plasma was diluted with an aqueous acidic solution and loaded onto the SPE cartridge previously conditioned with organic solvent and water. The plasma solution was then washed with a basic solution, eluted in solvent, evaporated to dryness, and reconstituted in mobile phase for analysis by LC-MS/MS. The extracted samples were analysed by reversed phase gradient chromatography employing an acidic aqueous buffer and acetonitrile as the organic modifier.
|
LC system: |
ACQUITY UPLC System with Binary Solvent Manager, Column Manager, and Sample Manager |
|
LC column: |
ACQUITY UPLC HSS T3 C18, 1.8 μm, 2.1 x 100 mm |
|
Column temp.: |
45 °C |
|
Gradient: |
30 to 95% organic over 3.5 min |
|
MS system: |
Xevo TQ-S |
|
MS mode: |
Positive ion electrospray MS/MS |
|
MS transition: |
535 ⇒ 328 |
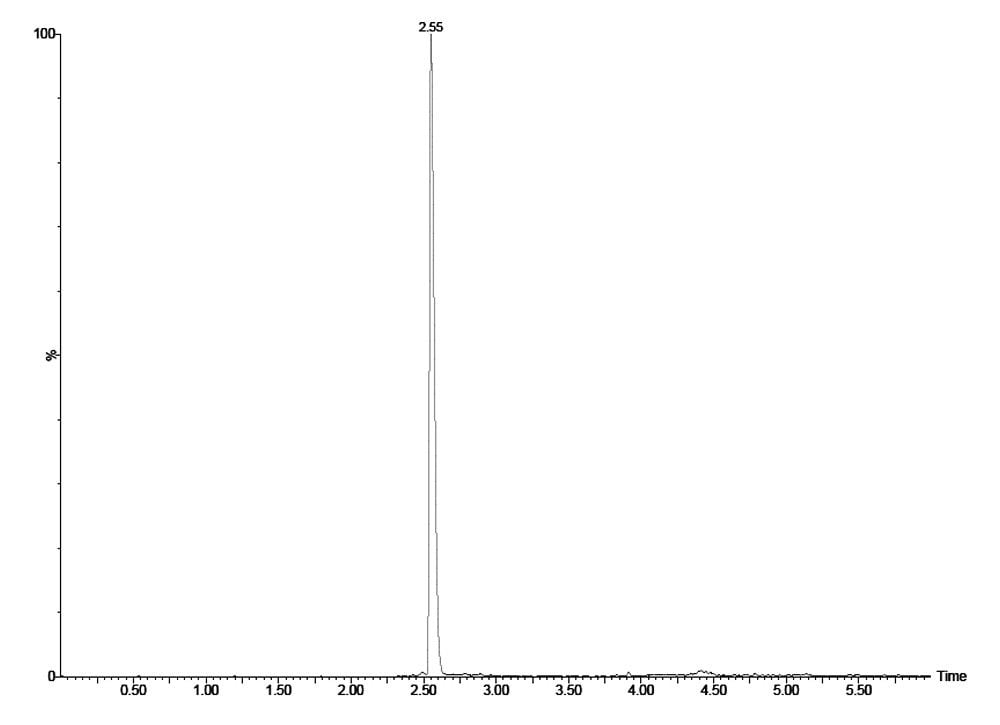
The chromatogram shown in Figure 2 shows the 47.5 pg/mL mid-level QC for desmopressin extracted from plasma, with the desmopressin analyte eluting with a retention time of 2.55 min. We can see from this data that the desmopressin peak shows excellent symmetrical peak shape and resolution from endogenous interferences. This peak shape and chromatographic resolution are due to the high resolution provided by the ACQUITY UPLC System and HSS T3 column combination.
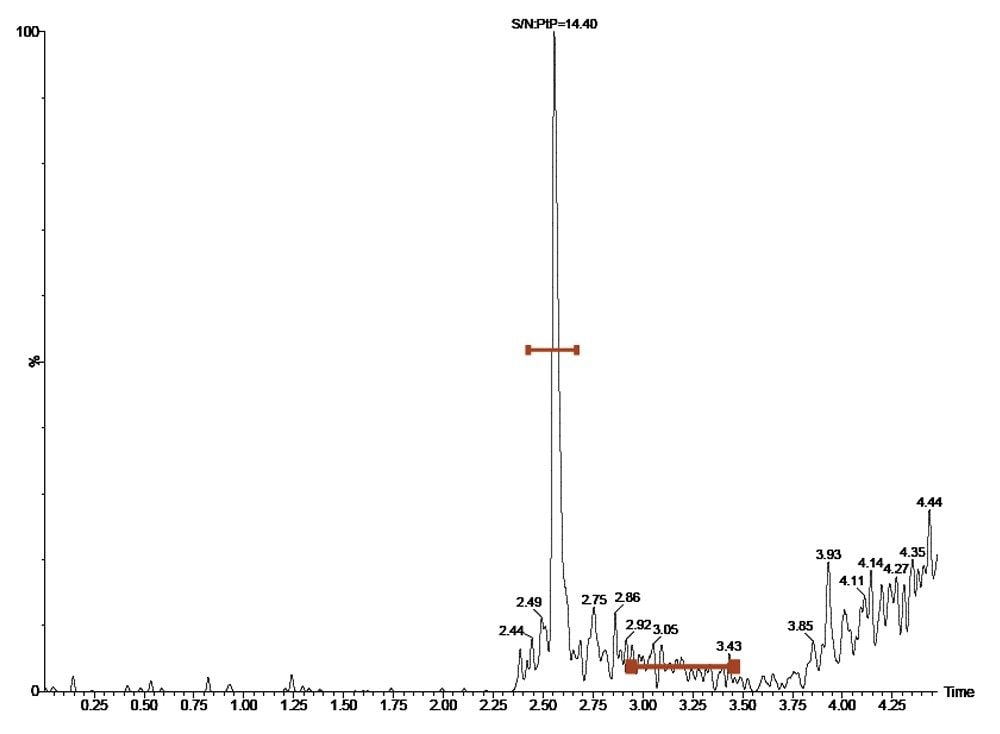
The lower level of quantification was determined to be 1 pg/mL, with a signal-to-noise of 14:1. The data displayed in Figure 3 shows the UPLC-MS/MS chromatogram illustrating the typical chromatogram from a 1 pg/mL standard of desmopressin in plasma. The peak area generated by the 1 pg/mL LLOQ standard was 181, which was nearly five times that obtained from the extraction of blank plasma.
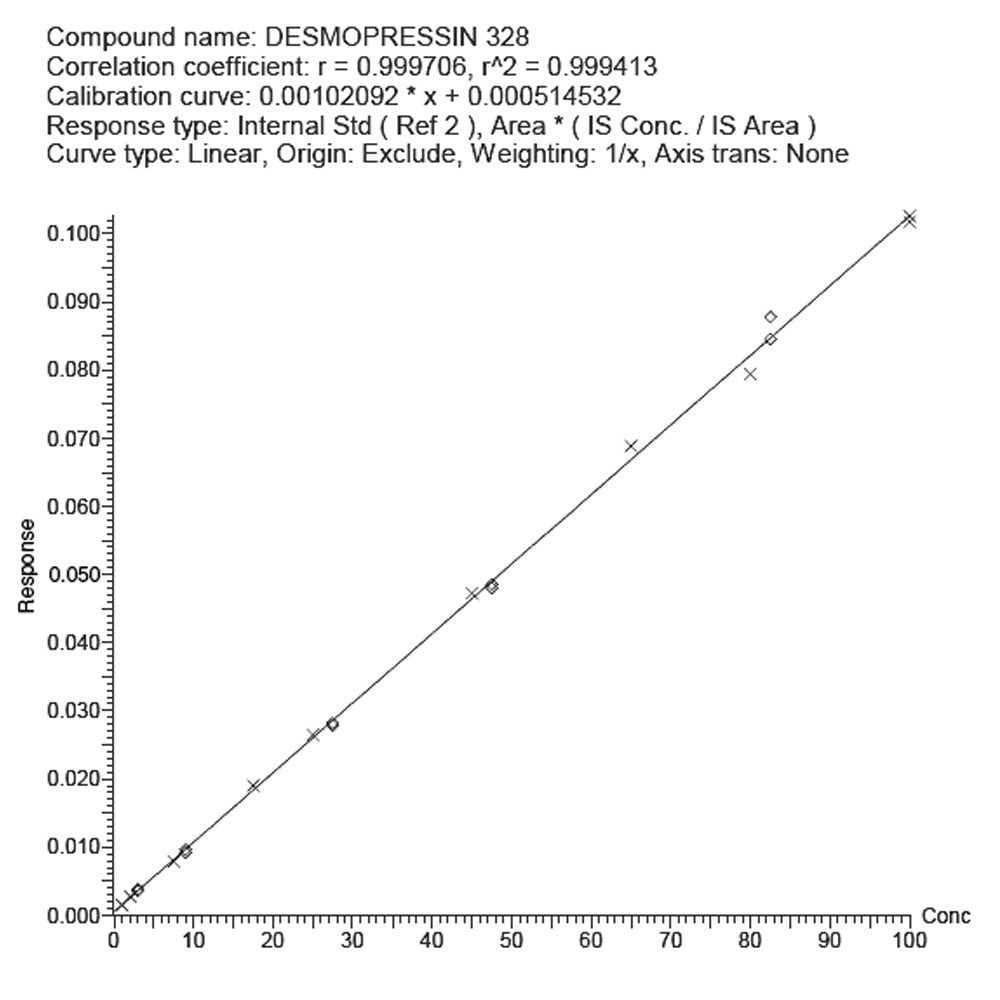
The low level of quantification obtained for this analysis was due, in part, to the high sensitivity detection provided by the Xevo TQ-S tandem quadrupole mass spectrometer. The co-joined off-axis StepWave ion guide in the Xevo TQ-S provides a high level of sensitivity while maintaining the robustness and cleanliness of the source and ion optics. This enables the Xevo TQ-S to increase the ion-flux entering the mass spectrometer and hence the sensitivity while maintaining usability of the instrument in terms of maximum up time. The assay was demonstrated to be linear over the range of 1 to 100 pg/mL; a typical calibration line is shown in Figure 4.
The low circulating levels of desmopressin, analogue of the human hormone vasopressin, requires a high-sensitivity assay to accurately define its pharmacokinetics in humans, especially pediatrics. The combination of Oasis SPE products, the ACQUITY UPLC System, and Xevo TQ-S Mass Spectrometer has facilitated the development of an assay for desmopressin with a LLOQ of 1 pg/mL in plasma. The assay showed excellent reproducibility, specificity, and robustness. Despite the complex nature of this analytical challenge, the overall analytical cycle time was just 6 minutes injection to injection.
720004056, August 2011