
In this application note we show a rapid analysis (<8.5 min) for 4-MEI and its isomer, 2-MEI using HPLC separation on a Waters CORTECS Column with mass detection.
Caramel colorings are complex mixtures that are added to a variety of foods and beverages such as sauces, candies, desserts, soft drinks, beers, and liquors. The coloring is produced by heating carbohydrates in the presence of ammonia or other reagents. A byproduct of this reaction is the compound 4-methylimidazole (4-MEI), that has been identified as a possible carcinogen in animal models, and has hence been listed as ‘possibly carcinogenic to humans’ by the IARC Monographs.1 The European Commission (EC) has therefore limited the concentration of 4-MEI in caramel colors to 250 mg/kg.2 In the US, the State of California has listed 4-MEI as a cancer-causing chemical on Proposition 653 and currently gives a “No Significant Risk Level (NSRL) of 29 μg per day.”
The recommended method for the analysis of 4-MEI and its related compounds is LC-MS, since photo diode array (PDA) detection does not provide sufficient sensitivity for detection of analytes at the levels found in beverage samples.4 The need to quickly obtain occurrence data for methylimidazoles in food has also driven the need for methods that do not require solid phase extraction (SPE) for sample cleanup or enrichment.4 In this application note we show a rapid analysis for 4-MEI and its isomer, 2-MEI using HPLC separation on a Waters CORTECS Column with mass detection.
During method development, it was noted that with an isocratic method, the system pressure increased following the analysis of soft drink samples. To ensure that all of the matrix components were removed from the column, a gradient method with a cleaning step was programmed, as shown above. Schlee et al. also commented on the requirement for ensuring the column was sufficiently flushed following the analysis of beverage samples.4 This provided a robust method for repeated analysis of all the beverage types shown in this application note.
|
LC system: |
Alliance |
|
Runtime: |
20.5 min |
|
Column: |
CORTECS HILIC 90 Å, 2.7 μm, 2.1 x 100 mm |
|
Mobile phase A: |
10 mM Ammonium formate pH-4 with formic acid |
|
Mobile phase B: |
Acetonitrile (0.1% formic acid) |
|
Injection volume: |
5 μL |
|
Sl.No |
Time |
Flow |
%A |
%B |
|---|---|---|---|---|
|
1 |
Initial |
0.4 |
7.5 |
92.5 |
|
2 |
8.5 |
0.4 |
7.5 |
92.5 |
|
3 |
9.0 |
0.4 |
60.0 |
40.0 |
|
4 |
11.0 |
0.4 |
60.0 |
40.0 |
|
5 |
11.5 |
0.4 |
7.5 |
92.5 |
|
6 |
20.5 |
0.4 |
7.5 |
92.5 |
|
MS system: |
ACQUITY QDa (Performance) |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
15 V |
|
Probe: |
600 °C |
|
Source: |
120 °C |
|
SIR imidazole: |
69.1 m/z |
|
SIR 2 and 4-MEI: |
83.1 m/z |
|
Acquisition: |
2 Hz |
Stock 1000 mg/L aqueous solutions of 2-MEI, 4-MEI, and imidazole were individually prepared. From these a mixed 10 ppm 2-MEI and 4-MEI standard, along with a separate 10 ppm imidazole standard were formulated. The 10 ppm mixed standard was diluted to prepare 10 individual standards with concentrations listed in Table 1. Each standard was fortified with 100 ppb imidazole as an internal standard. 100 μL of each standard was mixed with 900 μL acetonitrile prior to injection.
Three soft drink colas (two diet and one regular), along with an orange flavored soft drink, and two samples of whiskey were purchased in local stores. The soft drink samples were sonicated to remove carbonation. Each of the six samples was fortified with 100 ppb imidazole as an internal standard. Separate portions of each sample were fortified with 100 ppb 2-MEI and 4-MEI to determine recovery.
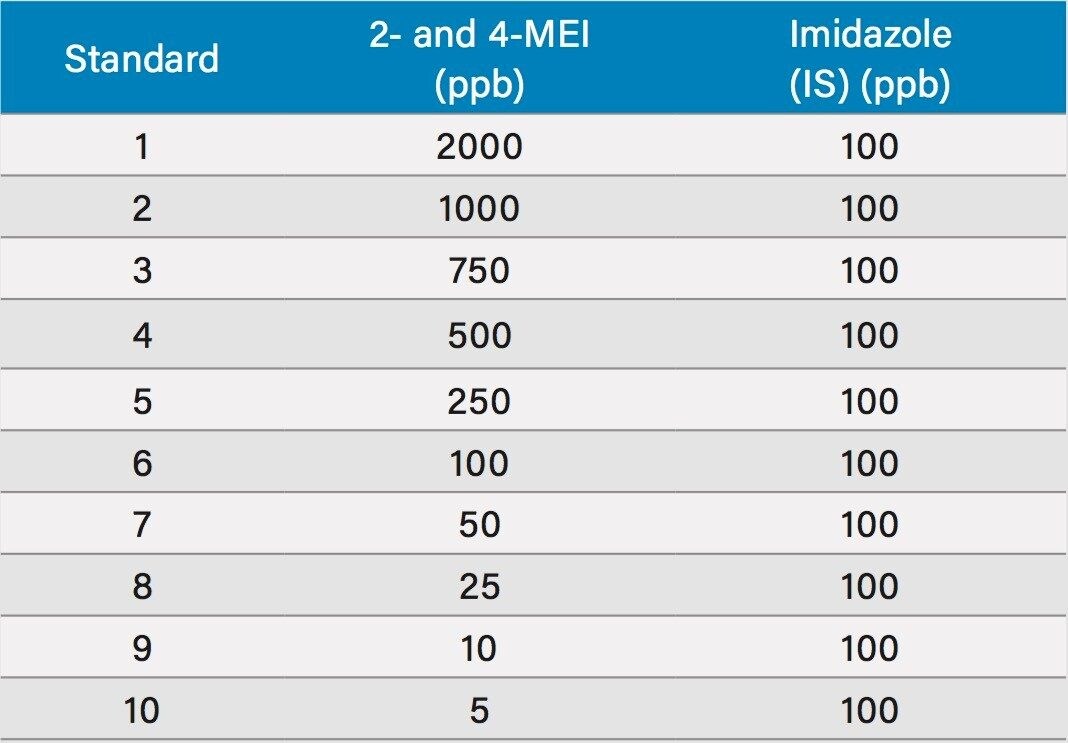
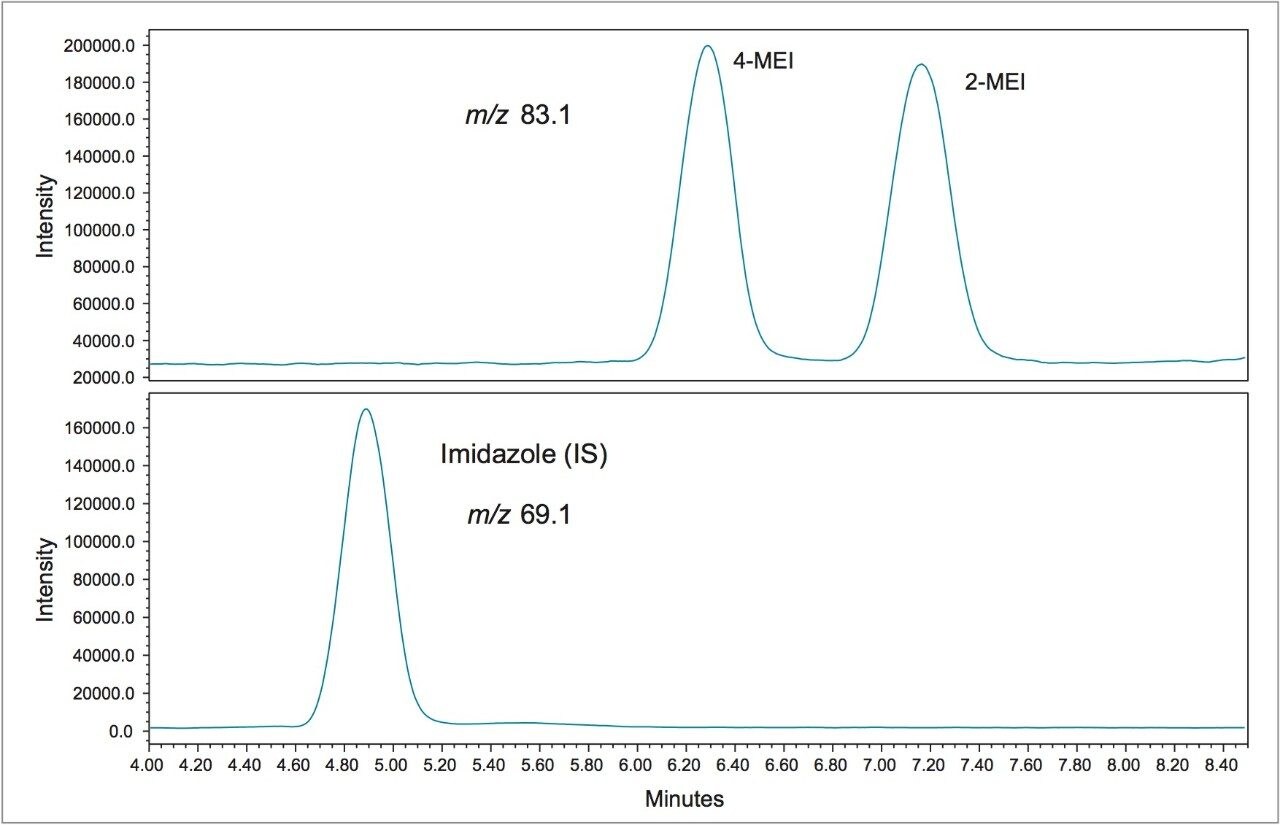
Figure 1 shows a chromatogram of 4- and 2-MEI at m/z 83.1 overlaid with the internal standard, imidazole at m/z 69.1. Excellent separation of the MEI isomers was achieved in less than 8.5 minutes. Figure 2 shows the calibration curve for 4-MEI. Linearity (R2) was 0.999 with residuals of <20% over the range of 50 to 2000 ppb. A chromatogram of the six samples studied is shown in Figure 3. 4-MEI was found in all of the cola samples, while 2-MEI was absent. Neither compound was found in the orange flavored soft drink as would be expected. A trace amount of both 4- and 2-MEI was found in one of the whiskey samples. Table 2 lists the amounts of 4-MEI found in each sample along with the recovery data.
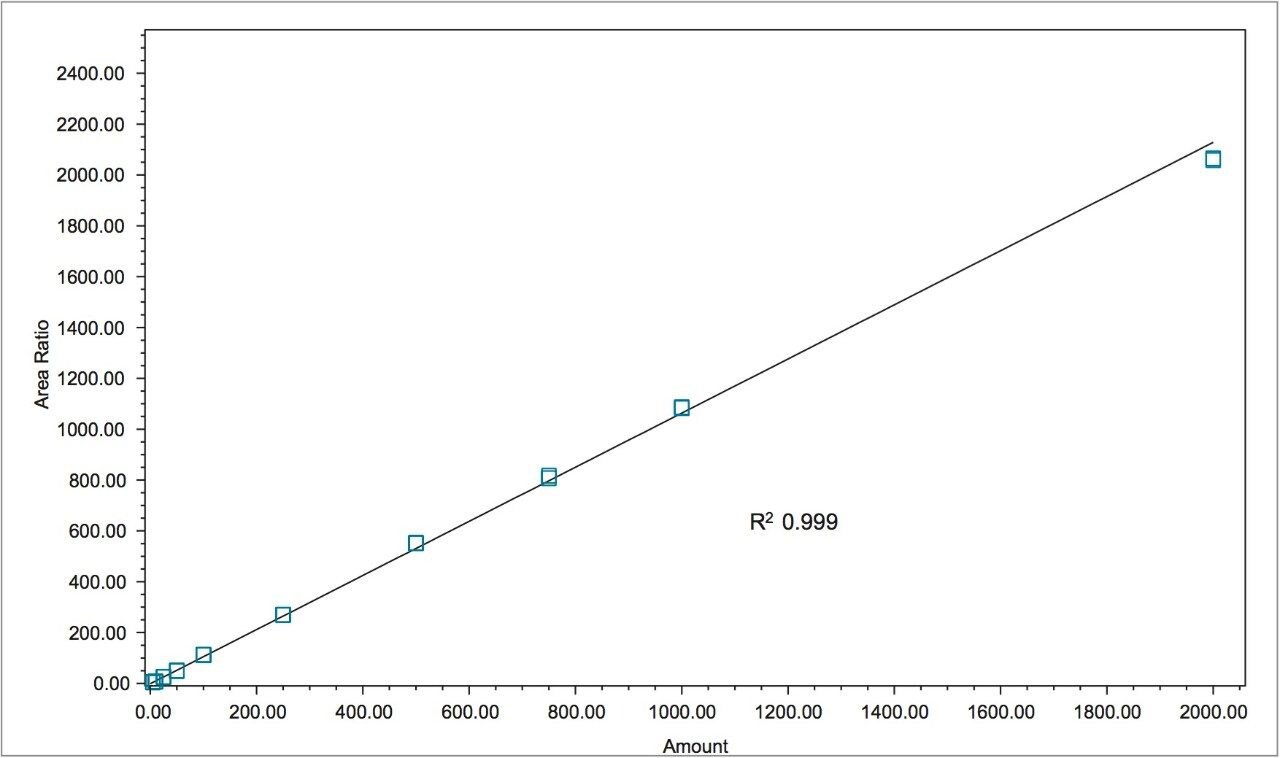
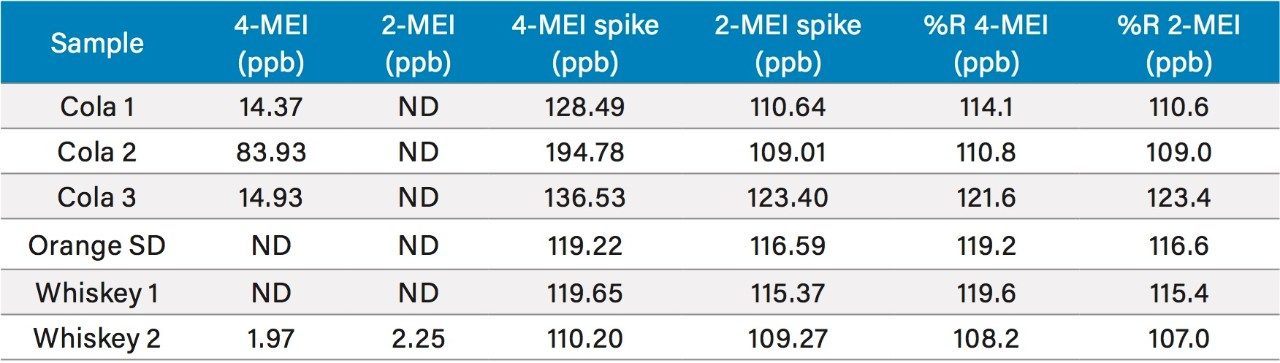
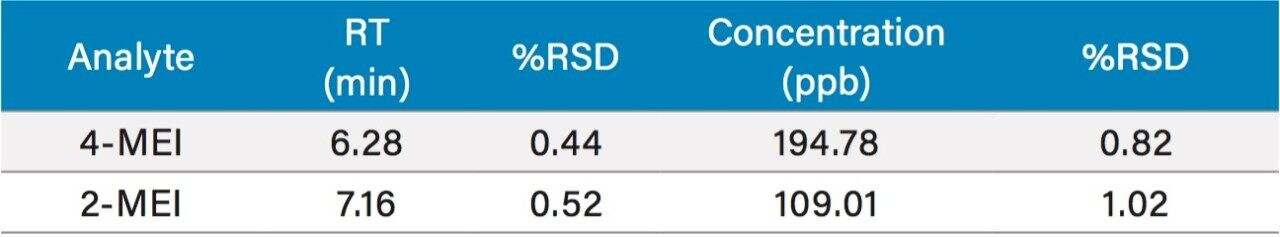
While one of the colas exceeded the California advisory guidelines for 4-MEI, all samples were well below the EU criteria of 80 mg/kg of body weight2 with no observed adverse effect level. Recoveries ranged from 110% to 123% for 2- and 4-MEI for all samples. Table 3 lists the reproducibility data for retention time and amount for seven injections of the spiked Cola Sample 2. The %RSD was <0.55% for retention time for both analytes, demonstrating that the column was properly equilibrated between injections. The %RSD for the calculated amount for the seven injections of 4-MEI was 0.82% and 1.02% for 2-MEI.
A rapid and simple gradient method for the determination of 4-MEI has been developed. 4-MEI, but no 2-MEI, was found in all of the colas sampled. A trace of both 4-MEI and 2-MEI was found in one of the whiskey samples. The orange soft drink sample did not contain either analyte.
The use of the Alliance HPLC System in combination with the ACQUITY QDa Mass Detector and CORTECS HILIC Columns provided multiple benefits, including:
720005200, November 2016