
For forensic toxicology use only.
This study reports a quantitative method based on SPE, following protein precipitation and UPLC-MS/MS. The method has been verified and its performance evaluated using authentic samples. Data were compared to results obtained with a GC-MS/MS method.
Cannabinoids should be monitored in both forensic and roadside drug testing laboratories, thus requiring an accurate, reliable, and robust method to quantify these compounds in biological samples. The developed approach meets these requirements, and demonstrates excellent correlation with an alternative GC-MS/MS method for the analysis of cannabinoids in human whole blood samples.
The method offers a number of noteworthy benefits over the GC-MS/MS approach including the following: utilization of UPLC rather than GC separation means that the lengthy post-extraction derivatization, used by the latter technique, can be eliminated with the analytical run time reduced from 20 minutes to 6.5 minutes, a three-fold reduction. The combination of these factors allows for significantly higher sample throughput. Furthermore, the superior sensitivity of the Xevo TQ-S permits detection of the required low levels of cannabinoids even with much smaller blood sample volumes, for example 0.2 mL compared with 1 mL required for other reported methods, even without the need of a post-extraction concentration step. This can be particularly advantageous as the volumes of whole blood available for testing can be small and must be sufficient for testing a number of drug classes.
Cannabis is the most widely used illicit substance in the world, and long-term use can lead to dependency. Consequently, the cannabinoids are one of the most commonly detected classes of illegal drugs, and their analysis plays a key role in both forensic and roadside drug testing.
Δ-9 tetrahydrocannabinol (THC) is the main psychoactive element present in the plant Cannabis sativa.1 THC produces a number of metabolites, including the active hydroxy-THC (THC-OH), and inactive carboxy-THC (THC-COOH), which can be detected circulating in blood after smoking or ingestion of cannabis.2,3 Quantitative analysis of the psychoactive constituents in whole blood is an indicator of cannabis consumption and may provide information relating to the individual’s state of impairment at the time of sample collection.
Previous publications have described the use of GC-MS, after solid-phase extraction (SPE),4 or liquid-liquid extraction,5 and pre-column derivatization for the determination of cannabinoids in whole blood. Recently a publication described the use of pre-column derivatization in conjunction with HPLC-MS/MS for this analysis.6 This study reports a quantitative method based on a previously reported SPE method following protein precipitation and UPLC-MS/MS.8 The method has been verified and its performance evaluated using authentic samples. Data were compared to results obtained with a GC-MS/MS method.
THC, THC-OH and THC-COOH (1 mg/mL), and their deuterated (d-3) analogues for use as internal standards (ISTD) at 0.1 mg/mL were purchased from LGC Standards (Teddington, UK). A mixture of pooled ISTDs at 50 ng/mL in methanol was prepared and stored at -20 °C.
Whole blood calibrators were prepared by spiking blank whole blood samples with known amounts of cannabinoids.
Forty-five anonymized samples containing pre-analyzed cannabinoids were obtained from J Monod Hospital, Le Havre, France. The samples were collected in the presence of either sodium fluoride or lithium heparin as anticoagulant.
Twenty microlitres ISTD were added to 0.2 mL whole blood (either sample or calibrator), which was then precipitated by drop-wise addition of 0.4 mL acetonitrile while vortex-mixing. The sample was then centrifuged at 4000 g for 10 minutes at 4 °C. Supernatant (0.4 mL) was then added to 0.6 mL 1% ammonium hydroxide, and the resulting solution loaded onto the Oasis MAX SPE Cartridge (p/n 186000366).
|
Condition: |
1 mL methanol followed by 1 mL 1% ammonium hydroxide |
|
Load: |
prepared 1 mL sample |
|
Wash: |
0.5 mL 50% acetonitrile |
|
Dry: |
10 minutes under full vacuum |
|
Elute: |
1.5 mL hexane/ethyl acetate/acetic acid (49:49:2 v/v/v) |
|
Evaporate: |
under nitrogen at 40 °C |
|
The sample was reconstituted in 0.133 mL 70% aqueous methanol, vortex-mixed, then transferred to a Waters Total Recovery Vial. |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 100 mm, (p/n 186002352) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
15 μL (PLNO) |
|
Strong wash: |
methanol/acetonitrile/ propan-2-ol (1:1:1 v/v/v) |
|
Weak wash: |
50% aqueous methanol |
|
Flow rate: |
400 μL/min |
|
Mobile phase A: |
0.1% formic acid |
|
Mobile phase B: |
acetonitrile |
|
Gradient: |
Linear from 60% B to 90% B over four minutes |
|
Mass spectrometer: |
Xevo TQ-S |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
2.5 kV |
|
Cone voltage: |
25 V |
|
Cone offset: |
50 V |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas: |
900 L/h |
|
Cone gas: |
150 L/h |
|
Acquisition mode: |
multiple reaction monitoring (MRM), see Table 1. |
MassLynx Software incorporating TargetLynx Application Manager
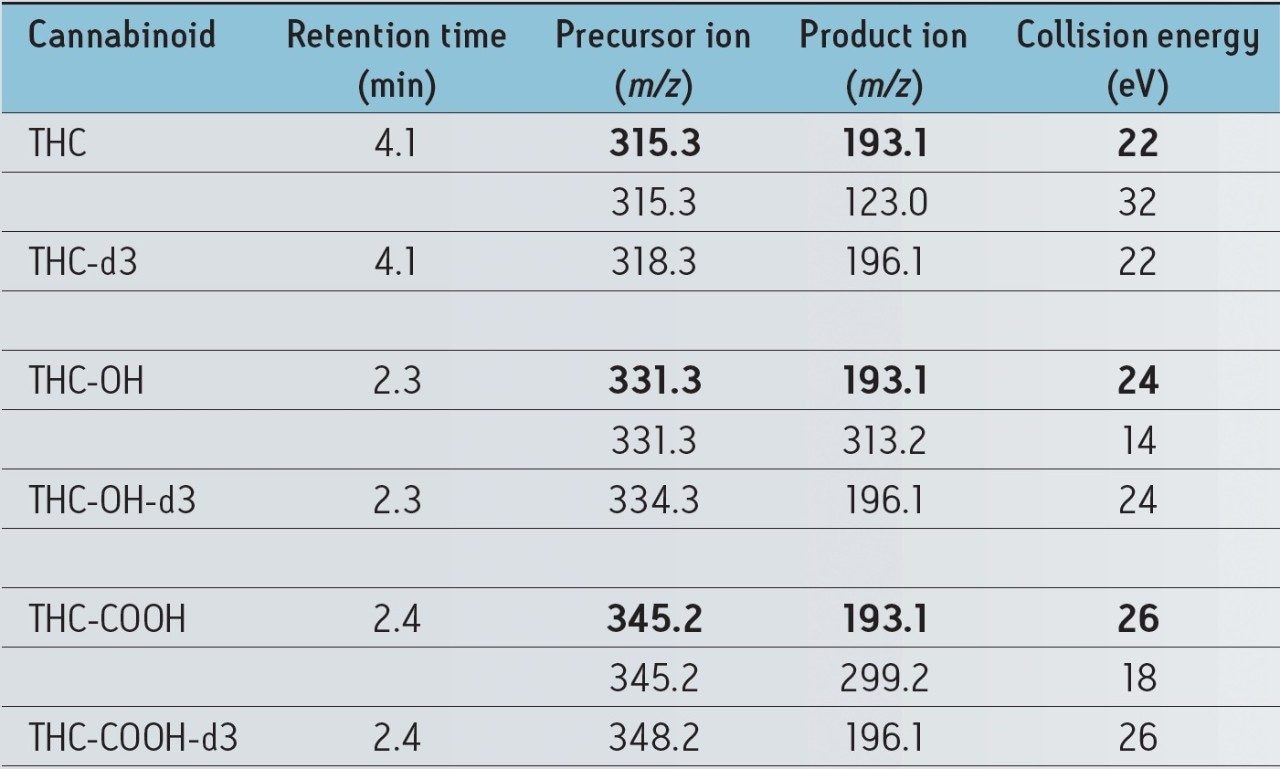
The MRM transitions for all of the cannabinoids and ISTDs are shown in Table 1. The analytes were monitored using two transitions (quantifier and qualifier). The acceptance criteria for a positive identification of analytes include retention time within 0.2 minutes of predicted, and the quantifier/qualifier ion ratio within 20% of the predicted ratio, which was based on the average of the ratios across the entire calibrator range. The ISTD was monitored using a single transition. Figure 1 shows a chromatogram of a whole blood calibrator spiked at 0.5 ng/mL.
To investigate linearity for all cannabinoids, spiked whole blood calibrators were prepared at 0.0, 0.5, 1.0, 2.5, 5.0, 10.0, 20.0, 30.0, 40.0, and 50.0 ng/mL. ISTDs were added, then the samples were precipitated and extracted as previously described, and subsequently analyzed by UPLC-MS/MS.
Quantification was performed by integrating the area under the peak for each analyte MRM trace, and referencing to the appropriate ISTD peak area. Data were processed using the TargetLynx Application Manager, and calibration curves plotted with a 1/x weighting. Inter-day coefficient of determination (assessed over five days) was >0.995 for each cannabinoid.
The limit of detection (LOD) was defined as the lowest concentration with a signal- to-noise ratio >10:1 (for both transitions) in spiked whole blood. The lower limit of quantitation (LLOQ) was defined as the lowest concentration with a signal-to-noise ratio>10:1 (for both transitions), and demonstrated a mean concentration bias <20% of target and %RSD of <20% in spiked whole blood. The LOD and LLOQ for each cannabinoid are summarized in Table 2.

Matrix effects and recovery (from six different sources of blank whole blood) were investigated at the following concentrations: 0.5 ng/mL (low), 5 ng/mL (medium), 25 ng/mL (high), and at 2.5 ng/mL for the ISTDs. Matrix effects were determined by comparing the peak areas obtained for blank whole blood spiked with cannabinoids after SPE, with peak areas obtained when spiked into 70% aqueous methanol. Recovery was determined by comparing cannabinoid peak areas from whole blood spiked pre-extraction with peak areas from whole blood spiked post-extraction. The results for each cannabinoid are shown in Table 3, whereby the values for matrix effects and recovery of the ISTDs matched those of the relevant cannabinoid.

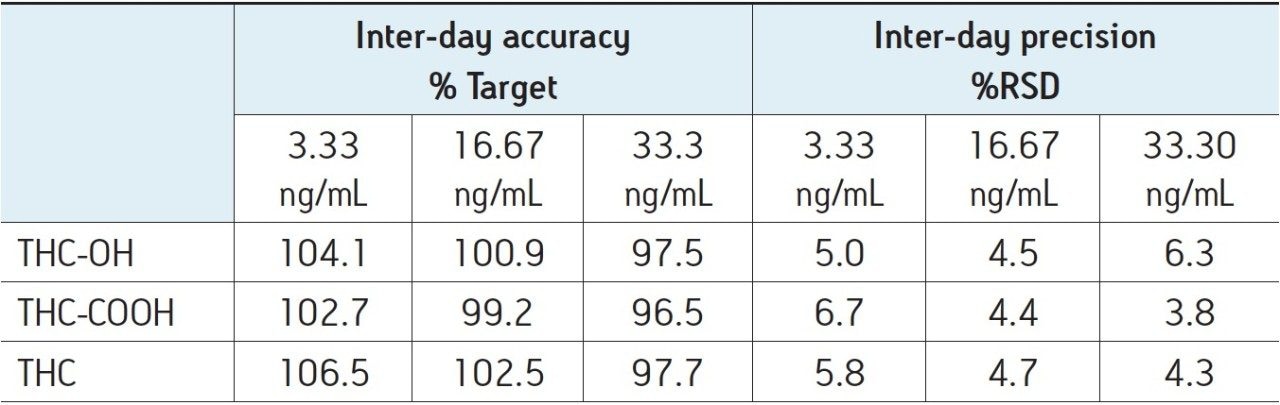
Inter-day accuracy and precision were assessed by analyzing three quality control (QC) concentrations (3.33, 16.67, 33.30 ng/mL) over five different days. The mean achieved values for the quality control replicates over the five-day period at the three concentration levels were within 20% of target, and the %RSD was <20%, as shown in Table 4.
Extracted sample stability was assessed by extracting multiple replicates of whole blood spiked at 2.5 ng/mL, pooling the reconstituted extracts, and storing the sample in the ACQUITY Autosampler at 10 °C. Injections were made every 60 minutes over the subsequent 24-hour period; no significant change in the peak area for the cannabinoids or ISTDs was observed.
Carryover of cannabinoids, following the injection of a 100 ng/mL spiked blood sample, was investigated and any cannabinoid observed was below the limits of detection.
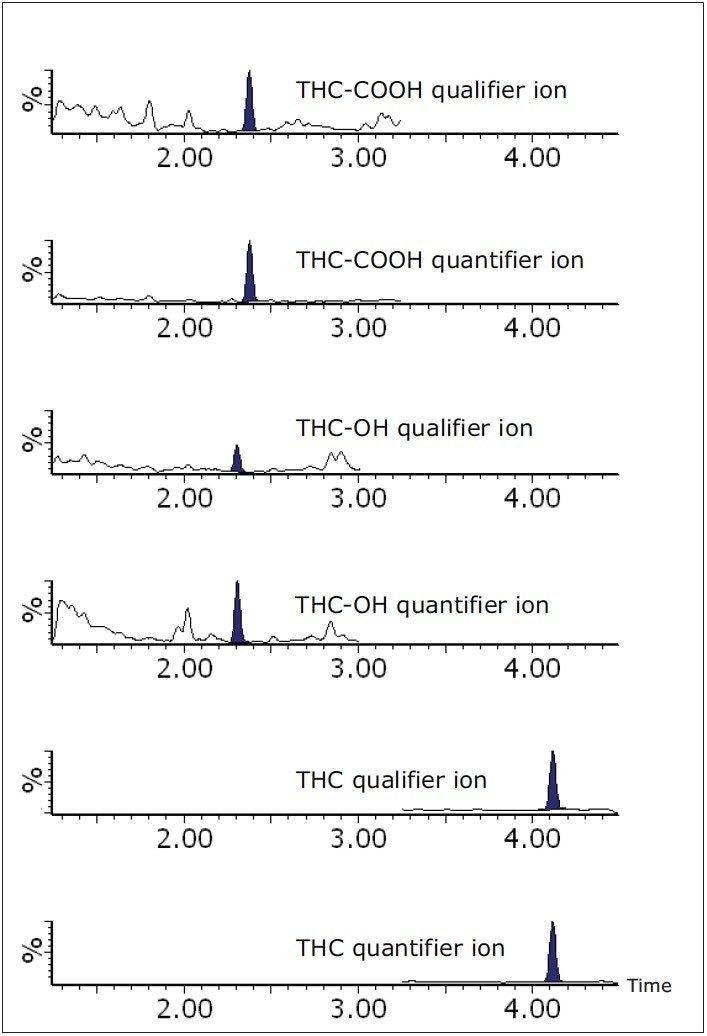
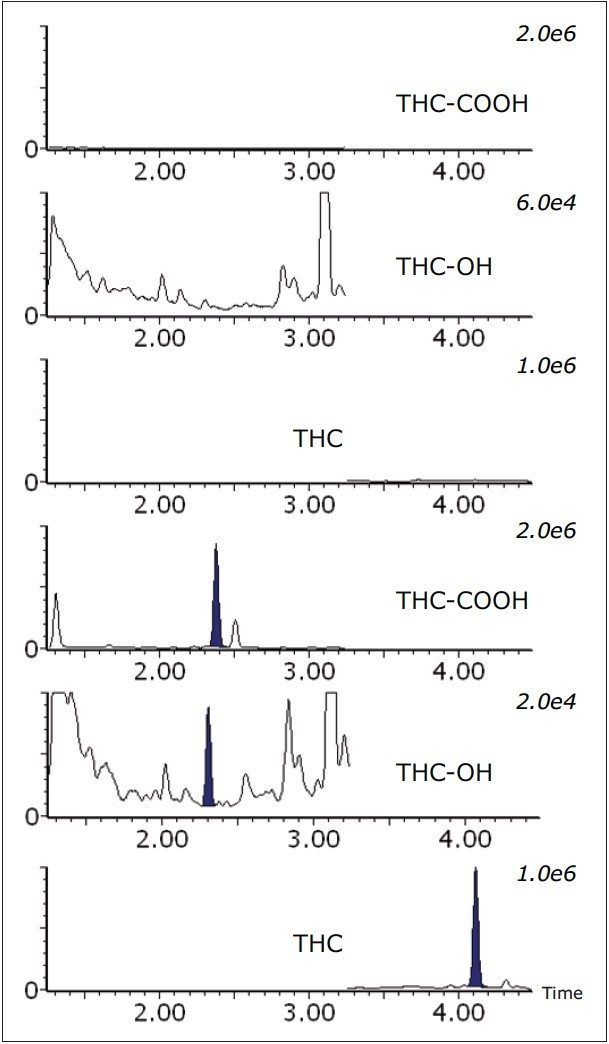
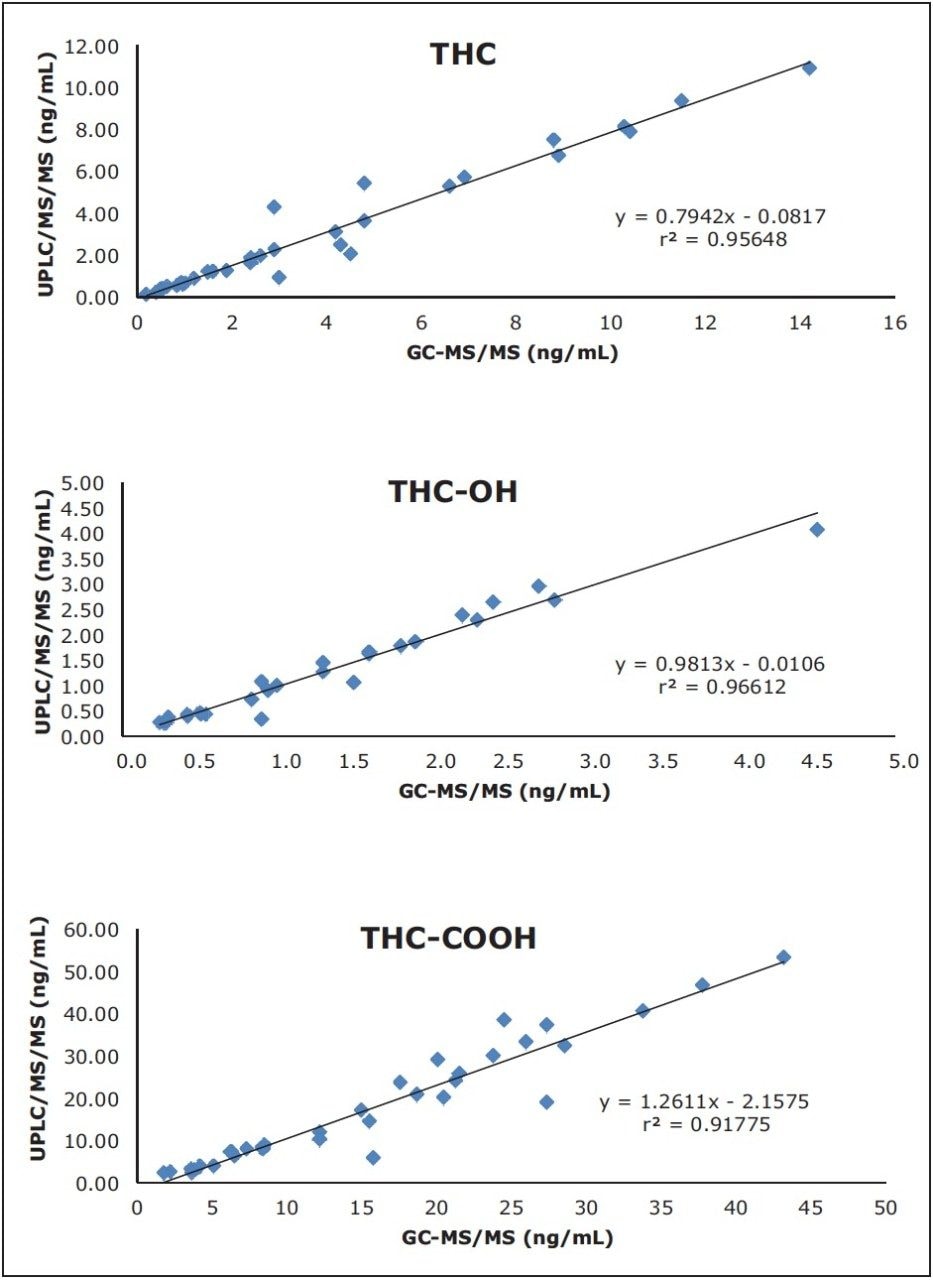
A total of 45 authentic whole blood samples were prepared and analyzed by UPLC-MS/MS, and the concentrations of detected cannabinoids calculated. Cannabinoids were detected in 35 of the 45 samples, passing both acceptance criteria for detection. A cannabinoid positive whole blood sample, shown in Figure 2, includes a cannabinoid negative sample for comparison. THC-COOH was present in all cannabinoid positive samples, while THC and THC-OH were found at levels above the LLOQ in 35 and 29 positive samples, respectively. These values were compared to those obtained at a separate laboratory using a GC-MS/MS method. The (r2) correlation values between the two data sets ranged from 0.9178 for THC-COOH to 0.9961 for THC-OH, as shown in Figure 3.
Cannabinoids should be monitored in both forensic and roadside drug testing laboratories, thus requiring an accurate, reliable, and robust method to quantify these compounds in biological samples. The developed approach meets these requirements, and demonstrates excellent correlation with an alternative GC-MS/MS method for the analysis of cannabinoids in human whole blood samples.
The method offers a number of noteworthy benefits over the GC-MS/MS approach including the following: utilization of UPLC rather than GC separation means that the lengthy post-extraction derivatization, used by the latter technique, can be eliminated with the analytical run time reduced from 20 minutes to 6.5 minutes, a three-fold reduction. The combination of these factors allows for significantly higher sample throughput. Furthermore, the superior sensitivity of the Xevo TQ-S permits detection of the required low levels of cannabinoids even with much smaller blood sample volumes, for example 0.2 mL compared with 1 mL required for other reported methods, even without the need of a post-extraction concentration step.4,5 & 7 This can be particularly advantageous as the volumes of whole blood available for testing can be small and must be sufficient for testing a number of drug classes. A full validation by the user would be necessary prior to adoption in a laboratory.
J Monod Hospital, Le Havre, France for supplying the anonymized authentic whole blood samples.
720004700, February 2014