
In this application note we describe the investigation of employing SFC/UV/MS for the quantitative analyses of (R, S)-goitrin in Isatis indigotica Fort extract and different Ban Lan Gen powder formulations. The SFC methodology was compared to the reported NPLC method in analysis time, LOQ, and LOD.
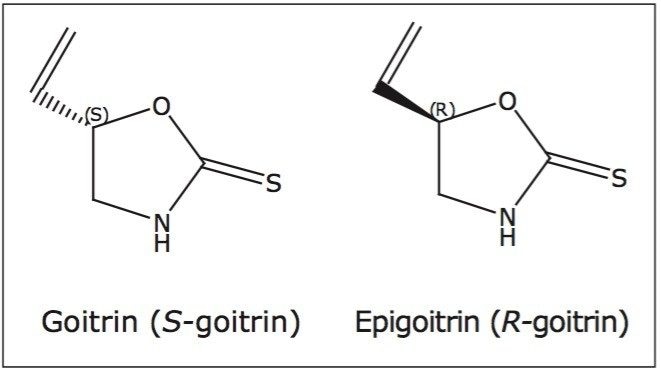
The therapeutic use of natural products dates back thousands of years, and continues to be an integral part of basic healthcare in many countries today. For example, TCM makes up half of the “basic” medicines mandated by the Chinese government for public use at all levels of its healthcare system.1 Since chirality is a fundamental characteristic of nature, it is not surprising that many of the therapeutic reagents in natural products or TCM are chiral. While one isomer could possess a desired therapeutic effect, its paired enantiomer could be inactive, have antagonist effects, or even have undesired effects. For example, Ban Lan Gen (the dried roots of Isatis indigotica Fort) is a commonly used TCM for treating fever and removing toxic heat. Pharmacokinetic studies indicate that the R-goitrin (epigoitrin) is one of the main constituents accounting for the antiviral activity of Ban Lan Gen.2-4 The S-goitrin (goitrin), however, is a potential goitrogen that causes an enlargement of the thyroid.5-6 It is, therefore, imperative to enantiomerically resolve R- and S-goitrin to better understand their respective pharmacological dose-response relationship and toxicity, for safe and effective use of the medicine.
Herein, we describe the investigation of employing SFC/UV/MS for the quantitative analyses of (R, S)-goitrin in Isatis indigotica Fort extract and different Ban Lan Gen powder formulations. The SFC methodology was compared to the reported NPLC method in analysis time, LOQ, and LOD.
|
Flow rate: |
3 mL/min |
|
Co-solvent: |
Methanol |
|
Temp.: |
40 °C |
|
Back pressure: |
120 bar |
|
Injection vol.: |
10 μL |
|
PDA: |
220 to 320 nm |
|
Gradient: |
20% to 20% in 2.0 min, 20% to 40% in 0.5 min, held at 40% for 3.0 min, 40% to 20% in 0.5 min |
|
Columns: |
(S, S)-Whelk-O 1 (4.6 x 250 mm, 10 μm) |
|
Ion source: |
APCI positive |
|
Corona current: |
10 μA |
|
Cone voltage: |
20 V |
|
Source temp.: |
150 °C |
|
Probe temp.: |
450 °C |
|
Mode: |
SIR @ m/z=130 |
|
Scan range: |
220 to 320 nm |
|
Resolution: |
1.2 nm |
|
Compensated wavelength: |
244 nm |
|
Compensated reference: |
270 to 320 nm |
|
Data rate: |
10 points/sec |
|
Filter constant: |
Normal |
All experiments were performed on a Waters Resolution SFC MS System controlled by MassLynx Software. The system consists of the following components: Fluid Delivery Module (FDM), Alias Autosampler, 10-port Analytical-2-Prep Column Oven, 2998 Photodiode Array (PDA) Detector, and 3100 MS Detector.
SFC-grade CO2 was from Air Gas (Salem, NH, USA). HPLC-grade water and diethyl ether were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLCgrade methanol and goitrin racemate, shown in Figure 1, were purchased from Thermo Fisher (Allentown, PA, USA). R-goitrin and dried roots of Isatis indigotica Fort were gifts from Prof. Zhengtao Wang at Shanghai Traditional Chinese Medicine University (Shanghai, China). Three different Ban Lan Gen powder formulations were purchased from a local Chinese herb store.
For dried roots of Isatis indigotica Fort and Ban Lan Gen powder formulations, 100 mg of the solid was sonicated in 5 mL of water for 1 hr, and allowed to sit for 1 hr. The sample was then centrifuged, and the supernatant was filtered through a 0.45-μm filter. 5 mL of diethyl ether was added to the filtered solution, followed by a liquid-liquid extraction. The procedure was repeated three times. The combined diethyl ether extract (a total of 15 mL) was dried down and reconstituted in 5 mL of methanol.
A goitrin standard solution of 0.005 mg/mL was prepared in methanol. Six replicates were performed for the repeatability studies. Inter-day repeatability was done in three days. Each day, six replicates were performed and the average peak area was used as one data point.
A goitrin stock solution of 0.1 mg/mL was prepared in methanol. The stock solution was serially diluted. For each data point, six replicates were performed, and the peak area was averaged.
A total of 0.14 mg of (R, S)-goitrin standard (0.07 mg for each enantiomer) was spiked into the dried root of Isatidis radix Fort in solid form. The mixture went through the same procedure as listed in “Sample preparation” before analysis. The ratio of the calculated “spike” and the true “spike” was defined as the recovery percentage.
An extensive chiral column screening using the goitrin racemate standard was conducted, and the (S, S)-Whelk-O 1 (4.6 x 250 mm, 10 μm) yielded the highest enantiomeric resolution. The method was further optimized for the Isatis indigotica Fort extract, primarily focusing on shortening the analysis time. The resulting chromatogram is shown in Figure 2 (A). Under the optimized condition, the R- and S-goitrin were separated from the sample matrix, while maintaining the enantiomeric resolution between the R- and S-goitrin. The total analysis time was 6 min. This represents nearly an eight-fold increase in speed, compared to the reported NPLC method.5 In SFC, a combination of supercritical CO2 and polar organic solvent(s), most commonly alcohol, are used as the mobile phase. Due to the inherent higher diffusivity and lower viscosity of supercritical fluid, it is not unusual that SFC provides a three- to eight-fold faster separation than NPLC.7
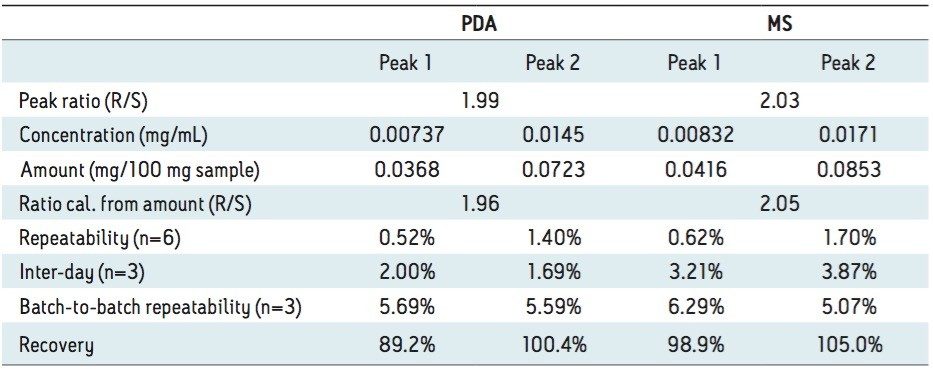
Figure 2 (B) shows the SFC/UV and MS chromatograms of the goitrin standards under the optimized conditions. The peaks were identified by running an R-enantiomer standard. Resolution, repeatability, inter-day stability (intermediate precision), LOD, and LOQ are summarized in Table 1.
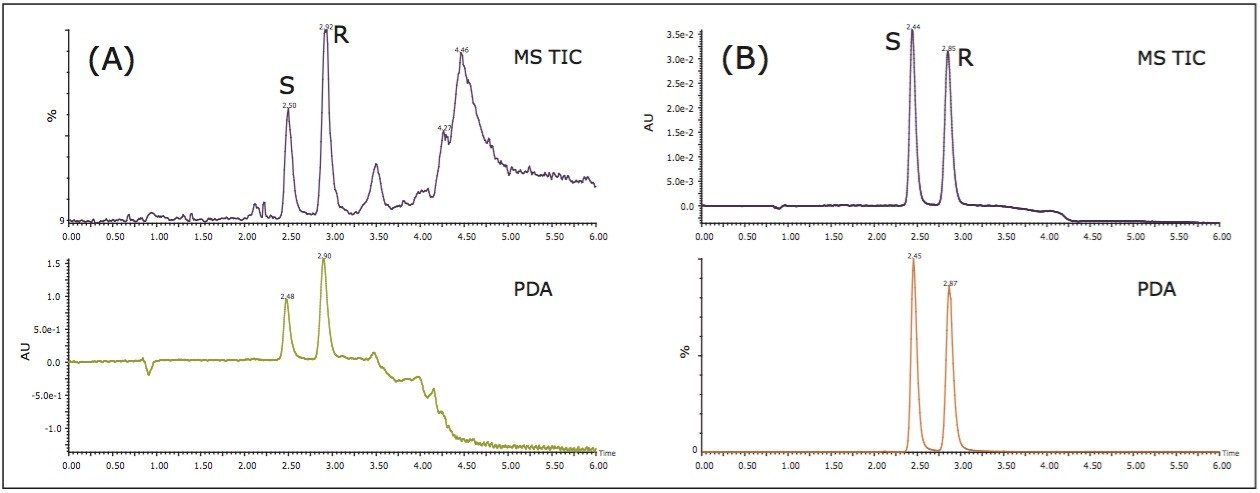
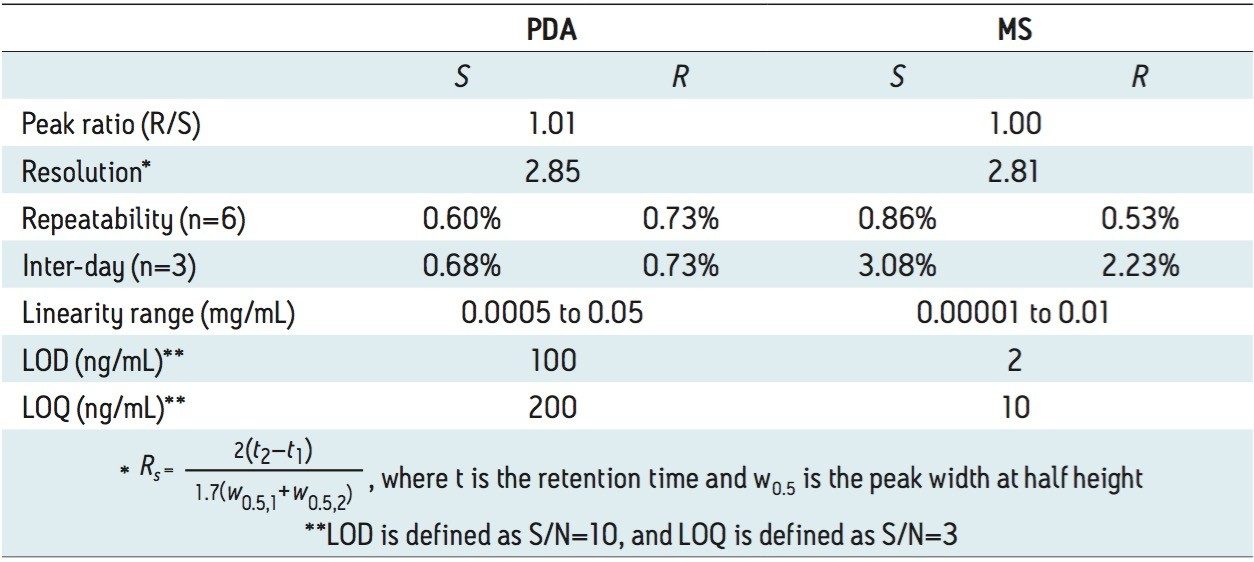
Repeatability with PDA detection was below 1%, and similar for both intra-day and inter-day experiments. Using MS detection, the inter-day variation was slightly higher than the intra-day variation.
The LOD and LOQ with PDA detection were one order of magnitude lower than those reported using an NPLC method.5 In our experiments, reference wavelength compensation was used in data acquisition. Reference wavelength compensation collects wide-band absorbance data in a region where the analytes have minimal or no absorption. The detector calculates the compensation value by averaging the absorbance values within the selected range of wavelengths. The averaged value is then subtracted from the absorbance value. Since the main absorbance (220 to 320 nm in our experiments) includes the reference bands (270 to 320 nm), noises from common sources, such as mechanical and thermal noise, can be effectively reduced; hence, increasing S/N.8
With MS detection, the LOD and LOQ were 2 and 10 ng/mL, respectively. At LOD, with a 10 μL injection, as little as 100 pg of each goitrin enantiomer was detected. This represents a three to four orders of magnitude improvement in detection sensitivity over the reported NPLC method with UV detection.5 This improvement, of course, arises from a more sensitive MS detection. However, in NPLC, hexane, heptanes, dichloromethane (DCM), isopropanol, and their mixtures are often used as the mobile phase. These solvents are not ideal, if not prohibitive, for MS detection. SFC, on the other hand, uses CO2 combined with MS-friendly alcohols, most commonly methanol, as the mobile phase. This, in turn, enables the incorporation of sensitive MS detection in SFC, often necessary for quantitative chiral analysis. SFC hyphenated with MS has indeed become a viable analytical tool in pharmaceutical research.9 Furthermore, the use of alcohol in SFC is more cost effective and environmentally sustainable than the use of hexane, heptanes, and halogenated solvents in NPLC.
Calibration curves for both UV and MS were constructed by analyzing the serially-diluted goitrin standards in triplicates. The results are shown in Figure 3. All calibration curves exhibited excellent linearity with the square of correlation coefficient (R2) above 0.999. There are also superb agreements between the R- and S-goitrin with both UV and MS detection.
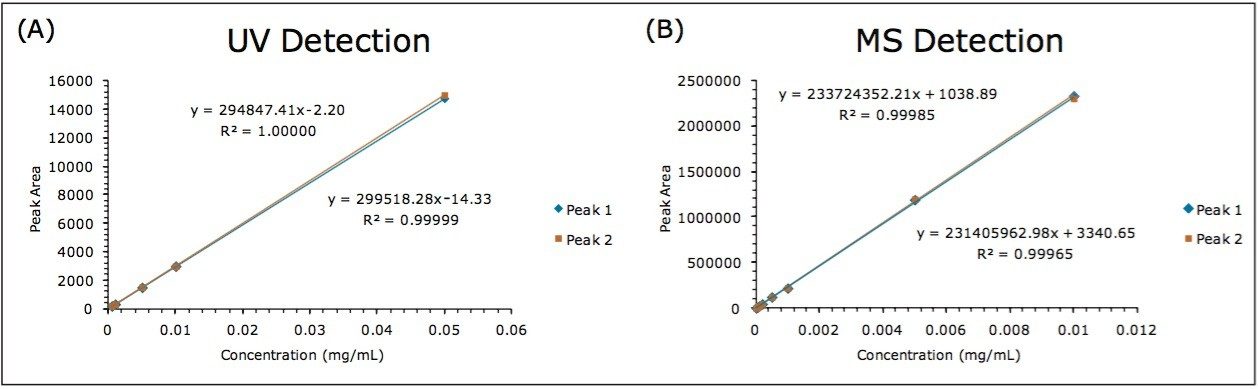
Table 2 summarizes the results of the analyses of Isatis indigotica Fort extracts. Compared to the results from the goitrin standards shown in Table 1, there are slightly higher variations with both UV and MS detection. It is also noted that batch-to-batch variation was consistently between 5% and 6%. This increased variation is likely due to the epiprogoitrin-epigoitrin transformations during the sample preparation procedure.
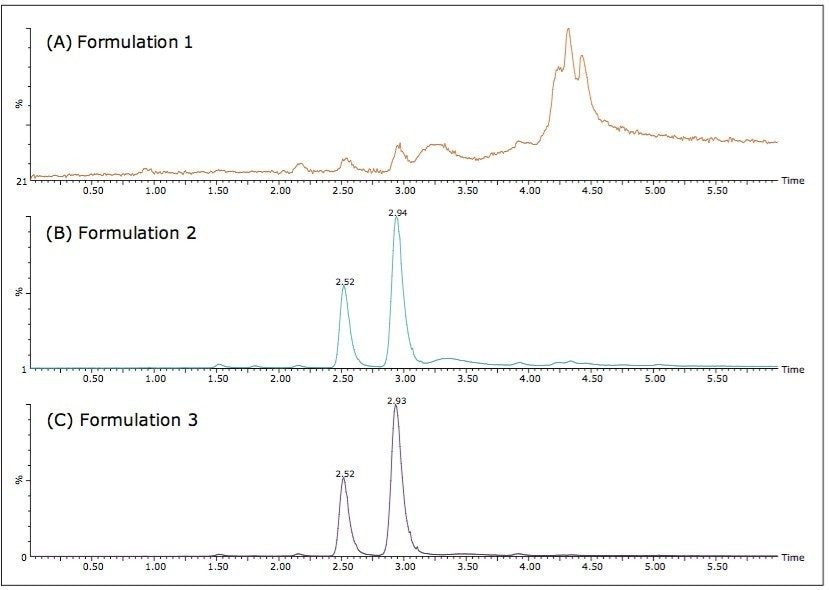
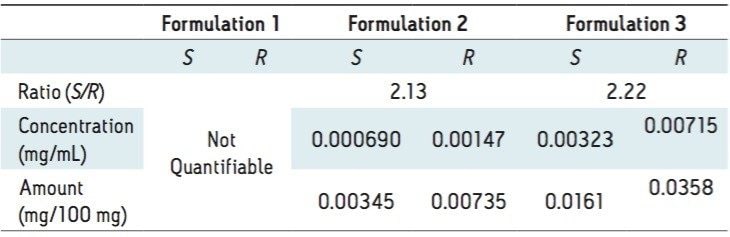
The three different powder formulations were all marketed as “Ban Lan Gen powder” by three different manufacturers. All of the powders are similar in appearance and tan in color. Figure 4 shows the SFC/MS chromatograms of the three formulations. Quantitative results are summarized in Table 3. It is evident that the three powder formulations differ substantially in goitrin content. Formulation 1 contains only detectable, but not quantifiable, goitrin, i.e. the concentration was between 2 to 10 ng/mL. Formulation 3 contains five times more goitrin than Formulation 2. It is also interesting to note that the R/S ratios are different between formulations 2 and 3. Currently, goitrin content is determined by reverse phase HPLC (RPLC)-based methodology10-11 where R- and S-goitrin are not resolved. Clearly, with varying R/S ratio evidenced in this study, the goitrin content cannot be accurately assessed via RPLC. Our observation underscores the importance of the enantiomeric resolution of R- and S-goitrin for better quantitation of the bioactive R-goitrin, better controlled pharmacological studies such as dose-response relationship and toxicity, and better quality control in Ban Lan Gen formulation manufacturing.
We described the development of an SFC/UV/MS-based assay for the qualitative and quantitative analyses of R- and S-goitrin. Under optimized conditions, the goitrin can be separated from the sample matrix while maintaining the enantiomeric resolution between the R- and S-goitrin. The total analysis time was 6 min, representing an eight-fold increase in speed, compared to the NPLC method. Excellent repeatability, intermediate precision, and linearity were achieved with the developed assay. With UV detection, the LOD and LOQ were one order of magnitude lower than those derived from NPLC/UV. With MS detection, the LOD and LOQ were three to four orders of magnitude lower than those from NPLC/UV.
The developed assay was then applied to the analyses of three different Ban Lan Gen formulations. The three powder formulations differ substantially in goitrin content. It is also noted that the R/S ratio varies from sample to sample. Our observation underscores the importance of the enantiomeric resolution of R- and S-goitrin for better quantitation of the R-goitrin, the active enantiomer contributing to the antiviral activity. SFC should be considered an integral part of the overall analytical platform for TCM and natural product research, especially in the area of chiral analysis.
720004206, January 2012