Tools to Evaluate the Impact of Re-Equilibration on a Compendial Method Using the Alliance™ iS HPLC System PDA Detector
Lise Gauthier, Paula Hong
Waters Corporation, United States
Published on May 6, 2025
Abstract
Gradient HPLC methods should include a re-equilibration period at the end where the system is returned to initial conditions and the column and system are equilibrated before the next injection is made. The re-equilibration conditions should be optimized to include both the dwell volume of the HPLC system and a period where the column is maintained at initial method conditions. Standard recommendations are 5–10 column volumes of mobile phase for re-equilibration of the column, not including the dwell volume of the HPLC system. However, in an effort to increase throughput, some methods may sacrifice this re-equilibration time.
In this study, the impact of dwell volume on re-equilibration was examined using a method with less than 5 column volumes of mobile phase for re-equilibration. The dwell volume of the Alliance iS PDA System was easily compensated for using the tools available within the Empower™ Chromatographic Data System and the Alliance iS Method Editor. With the re-equilibration time compensated for dwell volume, the results showed improved baseline stability and retention time precision over those obtained without dwell volume compensation.
Benefits
- The Empower Chromatographic Data System allows for a delay of the next injection, providing a regulatory compliant tool to increase equilibration time without changing the instrument method.
- The Alliance iS Method Editor with Gradient Start feature allows for dwell volume compensation without adjusting the gradient table.
- The Alliance iS PDA System meets the system suitability requirements of the USP Acetaminophen Tablets Assay with improved baselines and retention time precision observed with an increase in re-equilibration (to 7 column volumes).
Introduction
Gradient HPLC methods should include a re-equilibration period at the end where the system is returned to initial conditions and the column and system are equilibrated before the next injection is made. A general guideline of 10 column volumes of mobile phase has frequently been used for the re-equilibration of reversed phase methods.1 This guideline, however, may not produce optimal re-equilibration times. The re-equilibration time may be too long resulting in less throughput and increased solvent consumption. Alternatively, if the re-equilibration time is too short baseline instability, inconsistent retention times and poor peak shapes may occur. Re-equilibration is a function of both the system and the column. Ideally, re-equilibration includes both the time for the composition change set by the instrument method to reach the column and a period of time where the system is held at initial conditions until the column and system are sufficiently equilibrated before the next injection is made.
The time it takes for a composition change to reach the column is defined by the dwell volume of the system and the flow rate of the method. The dwell volume of a system is the volume between the pump and the head of the column. When running established methods having defined re-equilibration times, such as compendial methods, the dwell volume of the system should be taken into account. If these methods specify brief re-equilibration times, failure to compensate for dwell volume may produce inadequate re-equilibration which ultimately impacts the analysis.
The impact of dwell volume on re-equilibration was examined through the USP Acetaminophen Tablets Assay, a gradient method with a specified re-equilibration time equivalent to approximately 4 column volumes of mobile phase. The method was run on the Alliance iS HPLC System PDA Detector with and without dwell volume compensation and the results compared.
Experimental
Method: USP Acetaminophen Tablets Assay
Standard Preparation
A stock standard solution was prepared using acetaminophen purchased from MilliporeSigma. The acetaminophen was diluted with 90:10 water:methanol to a final concentration of 1 mg/mL.
A working standard containing 0.01 mg/mL of acetaminophen was prepared by diluting 1.0 mL of the stock standard solution to 100 mL with 90:10 water:methanol.
Sample Preparation
A stock sample solution was prepared by transferring ten acetaminophen tablets (160 mg/tablet) to a 200 mL volumetric flask and diluting to volume with 90:10 water:methanol.
A sample solution having a nominal concentration of 0.01 mg/mL was prepared by diluting 0.25 mL of the stock sample solution to 200 mL with 90:10 water:methanol. The sample solution was filtered (0.45 µm filter) before use.
LC Conditions
|
LC system: |
Alliance iS HPLC System PDA Detector |
|
Detection: |
PDA Detector with 10 mm flow cell |
|
Wavelength: |
243 nm |
|
Sampling rate: |
10 points/sec |
|
Vials: |
LCGC Certified Clear Glass, 12 x 32 mm, Screw Neck Vial, with Cap and preslit PTFE/Silicone Septum, 2 mL Volume, 100/pk (p/n: 186000307C) |
|
Column: |
XSelect™ HSS T3 Column, 3.5 µm 3.0 x 100 mm (p/n: 186004780) |
|
Column temperature: |
40.0 °C |
|
Sample temperature: |
15.0 °C |
|
Injection volume: |
10.0 µL |
|
Flow rate: |
0.500 mL/min |
|
Mobile phase A: |
1% glacial acetic acid in water |
|
Mobile phase B: |
Methanol |
|
Needle wash: |
60:40 methanol:water |
|
Seal wash: |
90:10 water:acetonitrile |
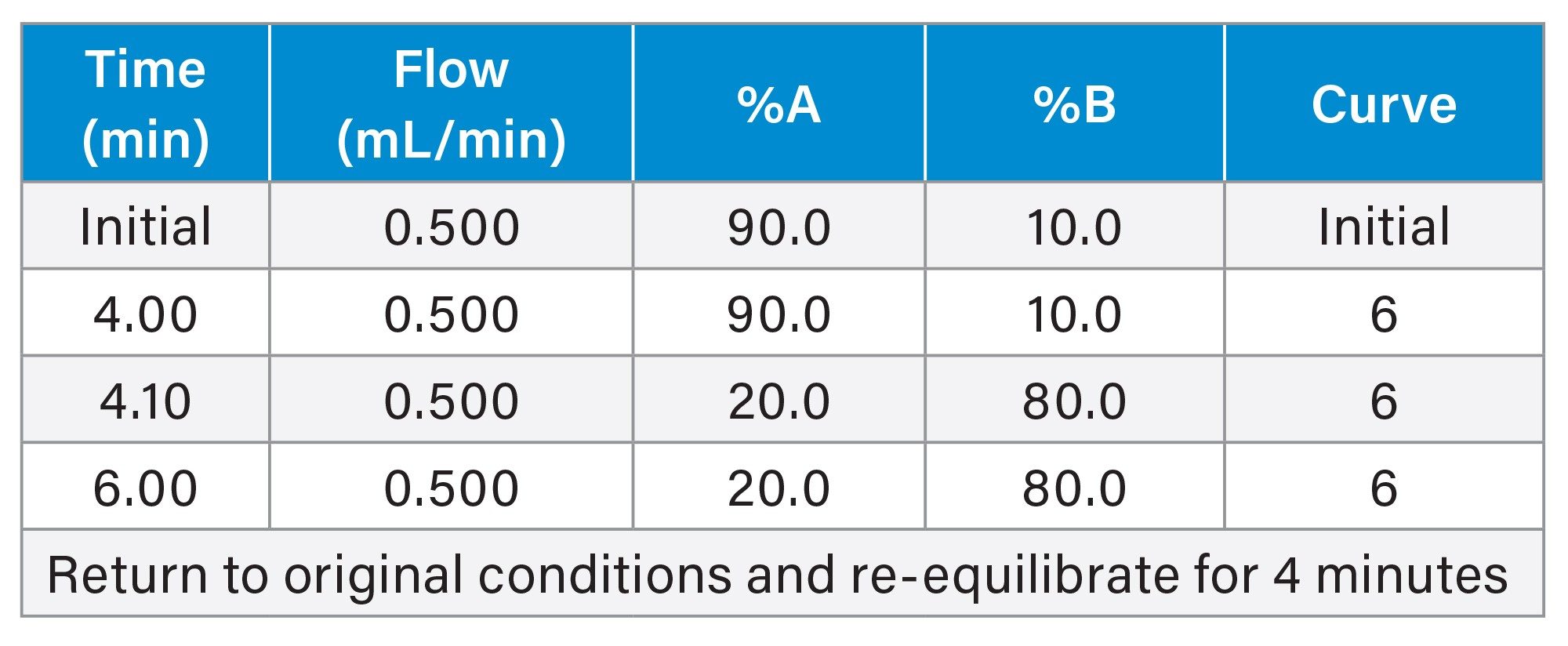
Gradient Table
Data Management
|
Chromatography data system: |
Empower™ 3.8.1 |
Results and Discussion
The impact of dwell volume on re-equilibration was assessed by running the USP Acetaminophen Tablets Assay on the Alliance iS HPLC System PDA Detector. The method is a reversed phase binary gradient with UV detection. The gradient involves an initial isocratic hold at 10% B for four minutes followed by a step to 80% B and a 2-minute hold at 80% B. The USP method specifically states to return to original conditions and re-equilibrate the system for 4 minutes.
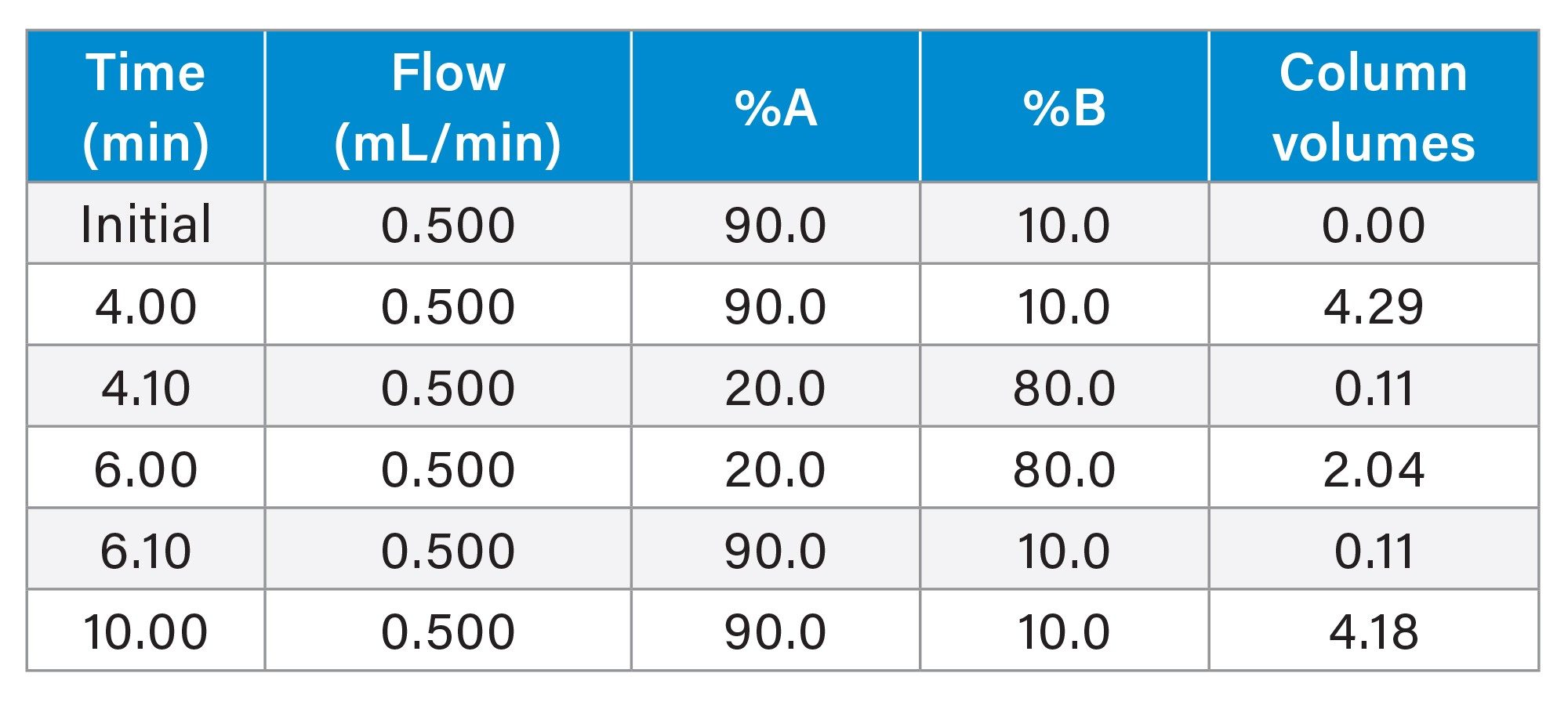
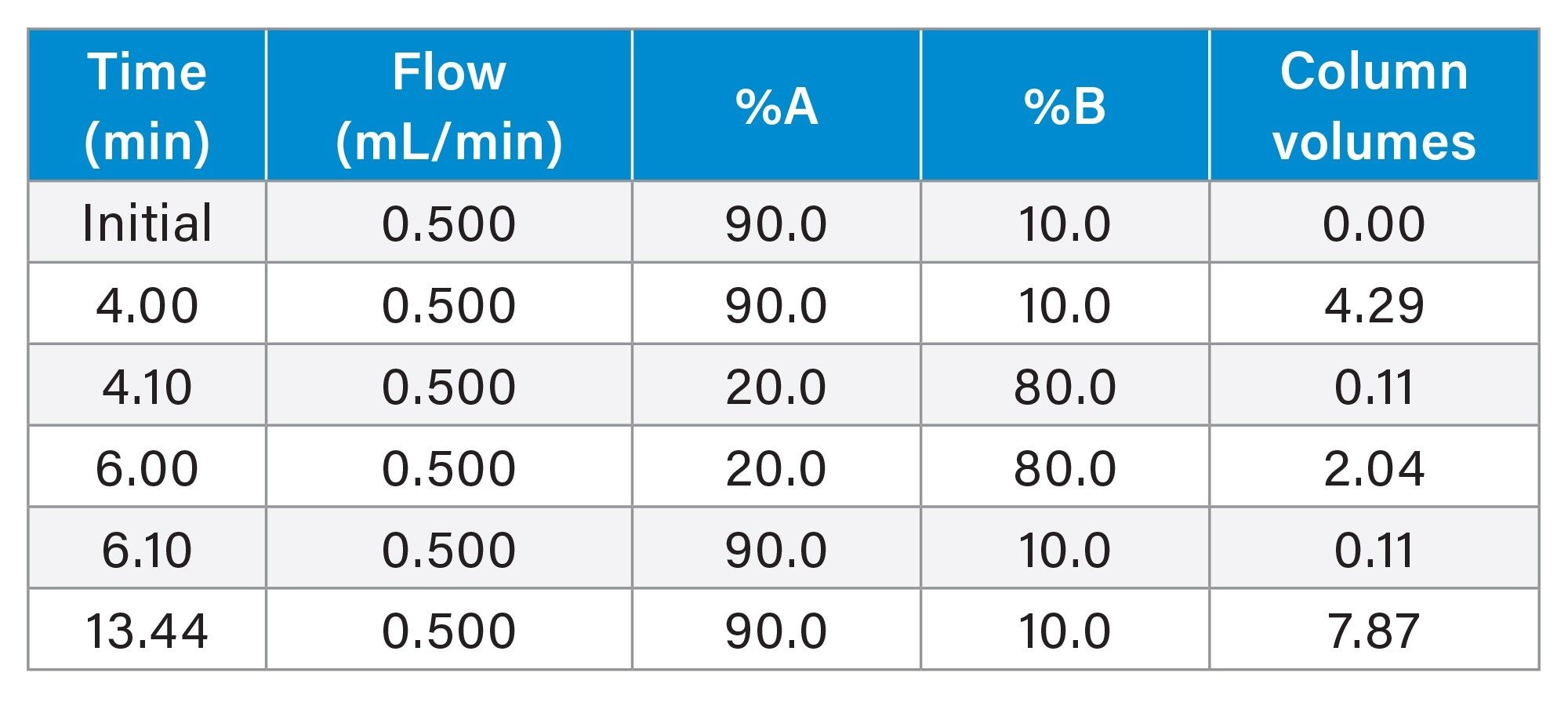
The method was run on the Alliance iS HPLC System PDA Detector without compensating for dwell volume in the re-equilibration time. The gradient used, including the number of column volumes of mobile phase per each step (calculated by the Waters Column Calculator) is presented in Table 1. Without dwell volume compensation, 4.18 column volumes of mobile phase are used for re-equilibration. (Note: One column volume is equivalent to 0.465 mL)
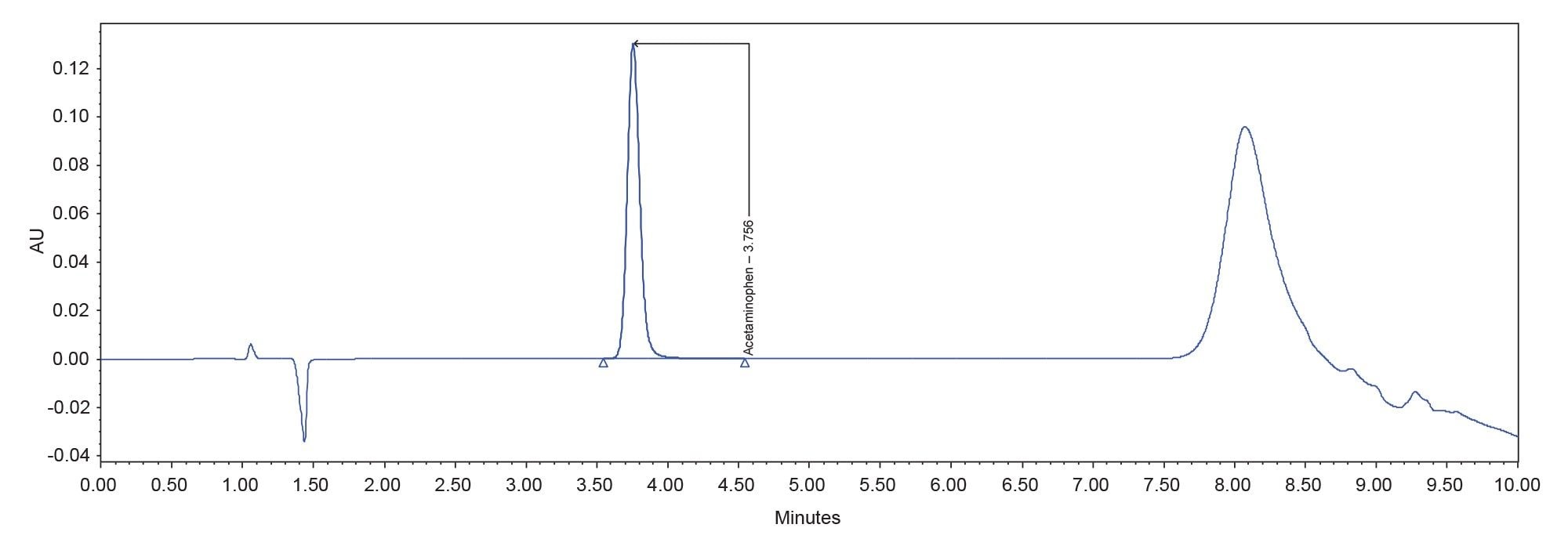
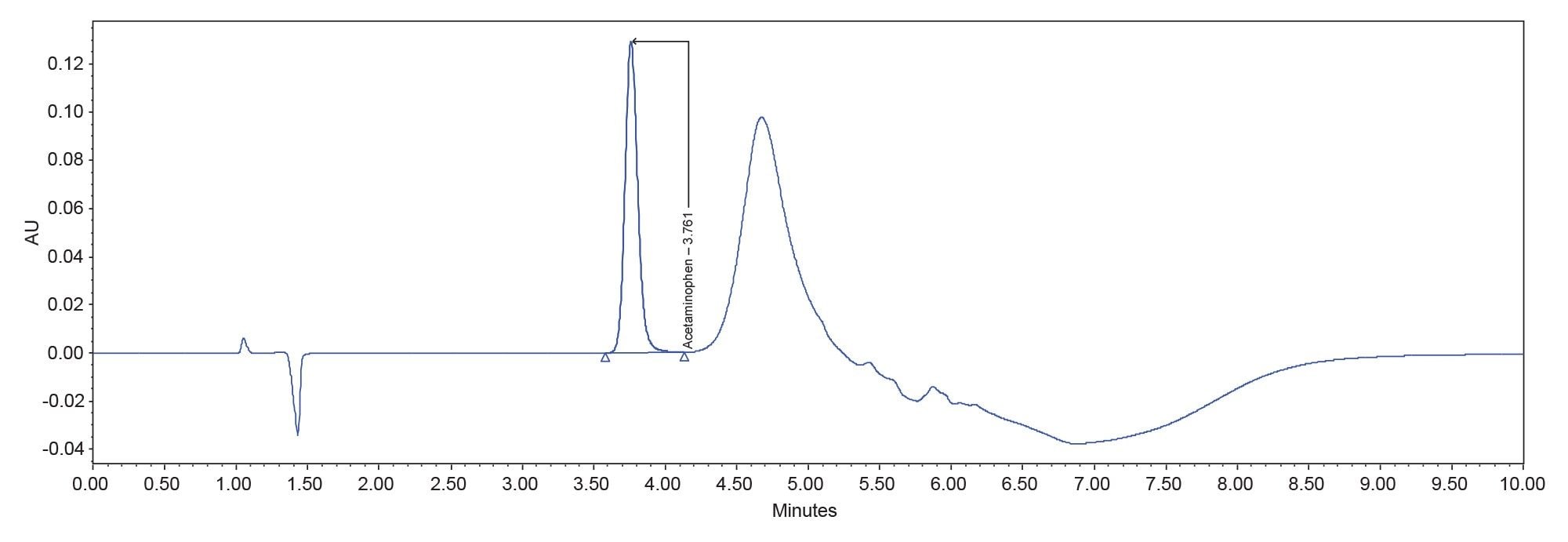
The results of the analysis are summarized in Table 2. The USP system suitability criteria were met, and the sample found to contain 92.4% of the labeled amount of acetaminophen. A representative chromatogram of the working standard is found in Figure 1.
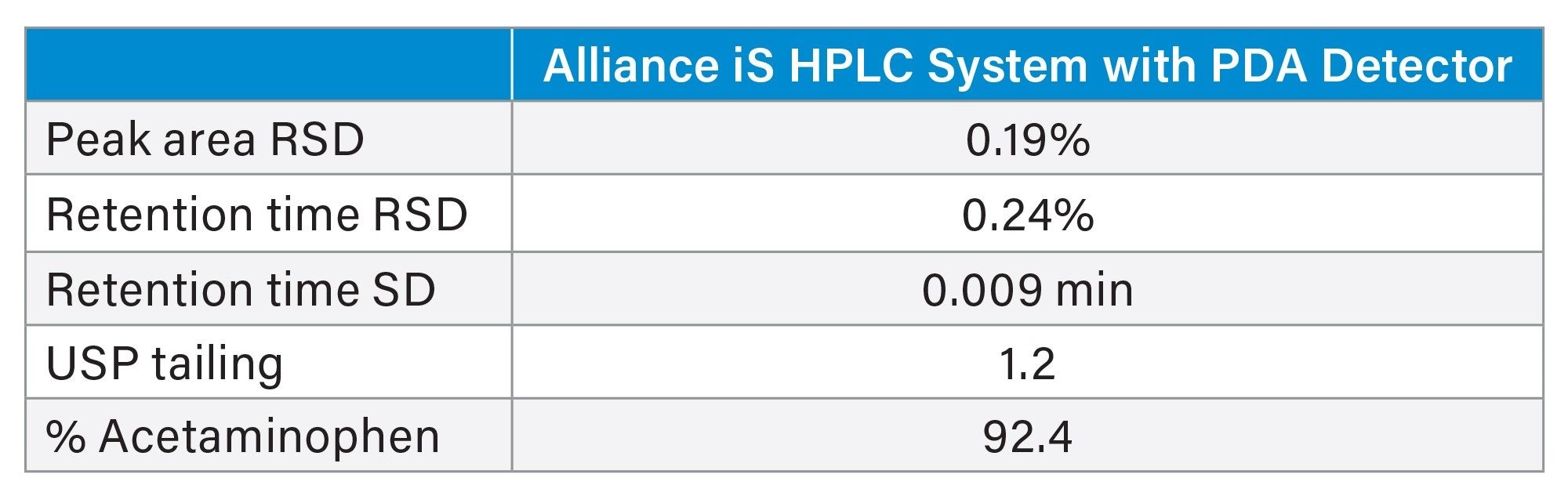
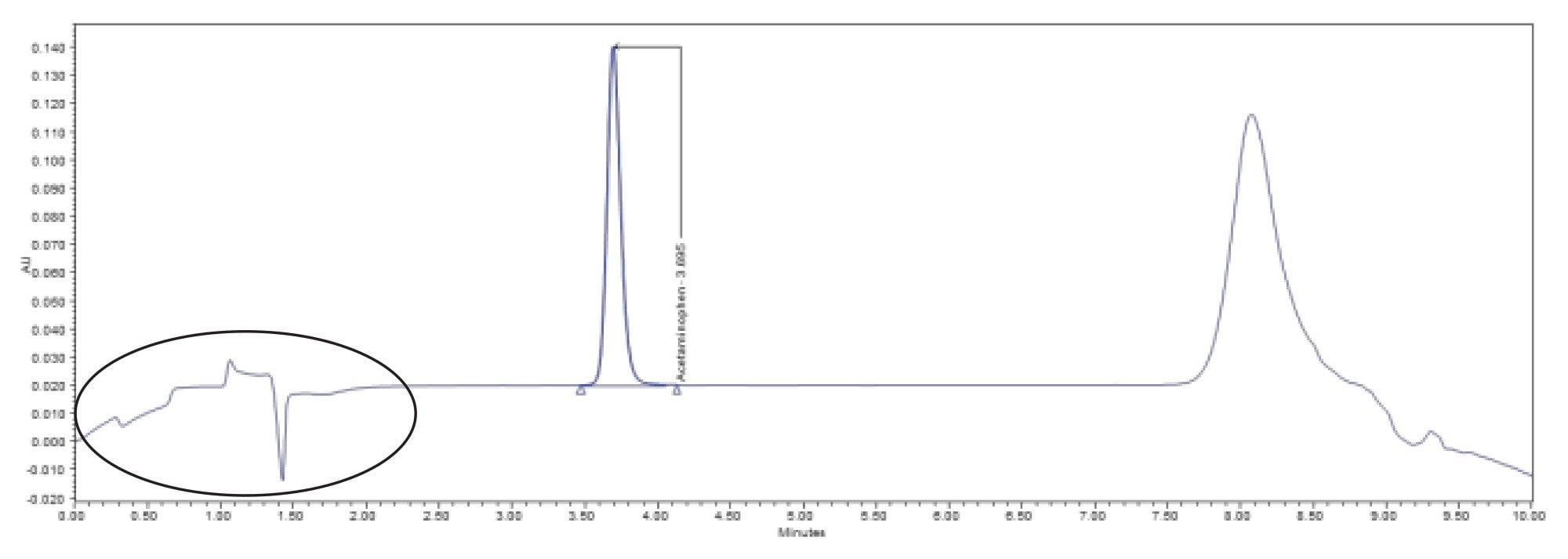
Although the system suitability criteria were met, a closer look at the data shows some irregularities. A review of the system pressure plots showed that the system failed to return to initial pressure before the next injection was made indicating that the system was not re-equilibrated (Figure 2). This inadequate re-equilibration time is also reflected in the baseline instability seen at the beginning of the chromatogram (Refer to Figure 1).
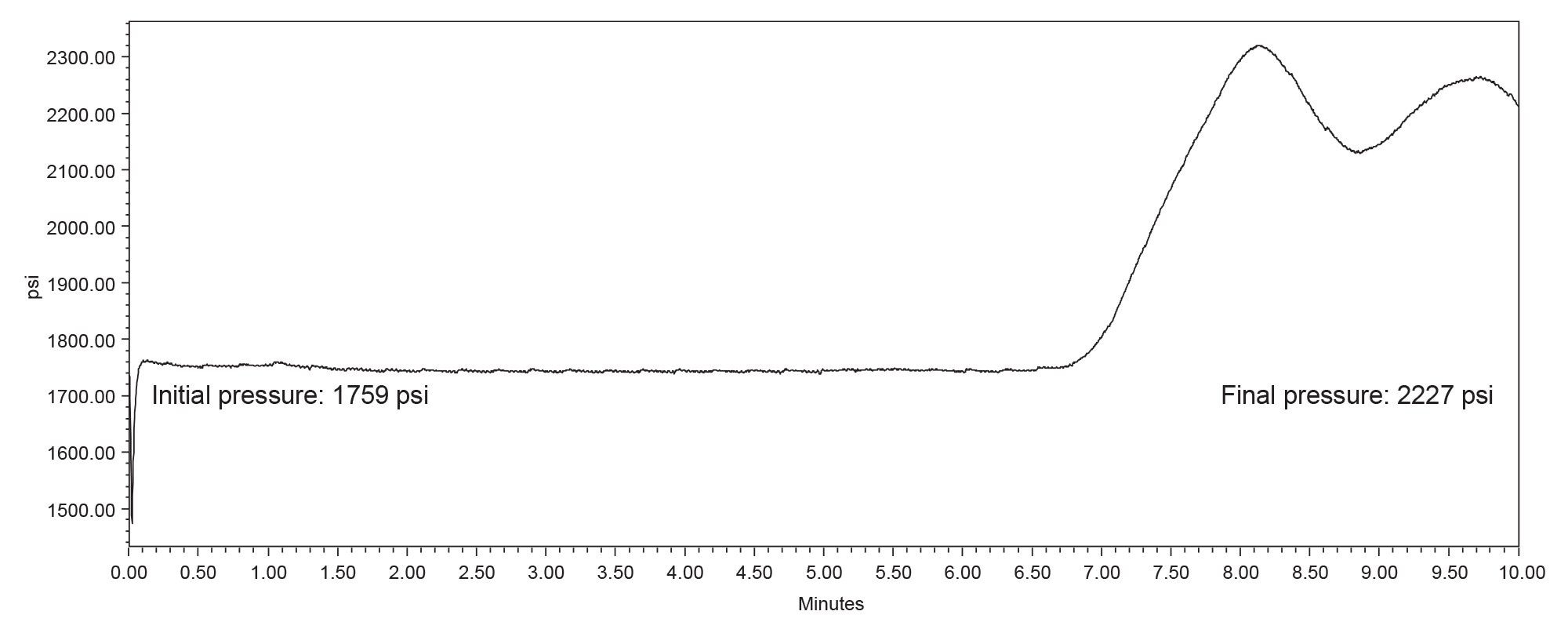
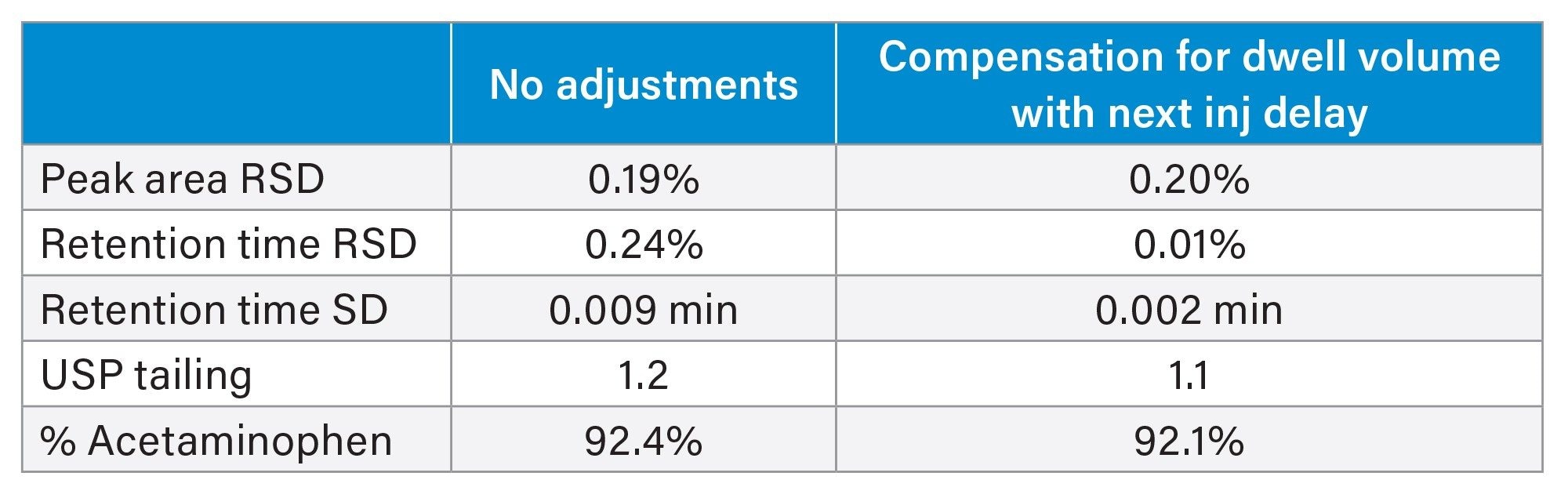
To investigate the impact of re-equilibration on the analysis, the method was run with dwell volume compensation by using features available in the Empower Chromatography Data System and the Alliance iS instrument method. The Empower Chromatography Data System has a feature which enables a delay of the subsequent injection, allowing for an additional equilibration hold at the end of an injection. Next Injection Delay holds the system at initial conditions for a time defined in the sample set. Alternatively, the gradient start option within the Alliance iS instrument method compensates for dwell volume by adjusting the gradient start time relative to the injection.
In this example, the dwell volume of the system was measured at 1.67 mL. Since the flow rate of the method is 0.5 mL/min, the time required for the system to return to original conditions is 3.34 minutes. This time was entered into the Next Injection Delay field of the Empower sample set and the analysis carried out. With the 3.34-minute delay employed, the system re-equilibration time was effectively 7.34 minutes, equaling 7.87 column volumes of mobile phase (Refer to Table 3).
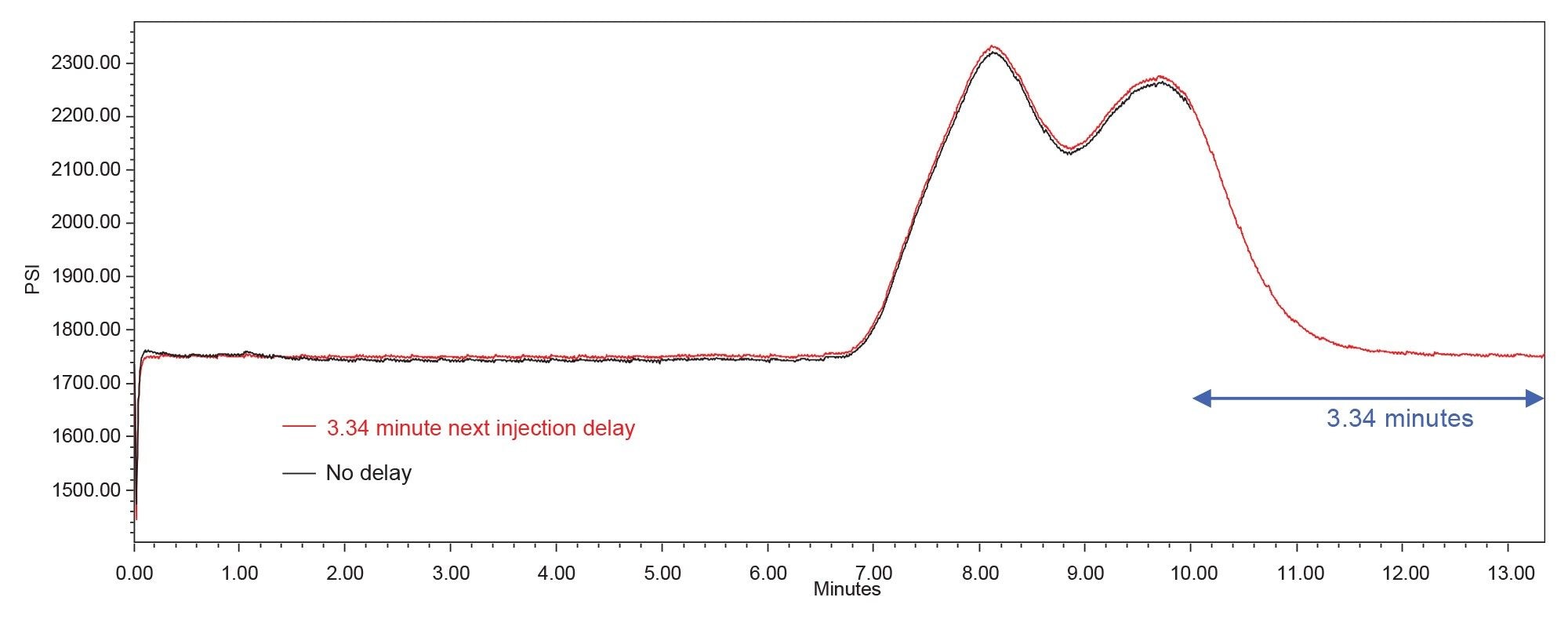
With the next injection delay employed, the system was held at initial conditions for 3.34 minutes before the injection was made. This allowed the system pressure to return to initial levels and maintain initial conditions before the next injection was made (Figure 3). Additionally, the baseline instability that was previously seen in the chromatograms disappeared (Figure 4). The results obtained using the 3.34-minute next injection delay are summarized in Table 4. The USP system suitability criteria were met. A comparison of these results to those obtained without dwell volume compensation show improved retention time precision with the 3.34-minute next injection delay employed. The results for peak area precision, tailing, and % acetaminophen are comparable to the results obtained without dwell volume compensation.
The Alliance iS Gradient Start feature was also used to compensate for dwell volume. From the instrument method, the gradient was set to start 1.67 mL before the injection. The results using Gradient Start are shown in Figures 5 and 6 and summarized in Table 5.
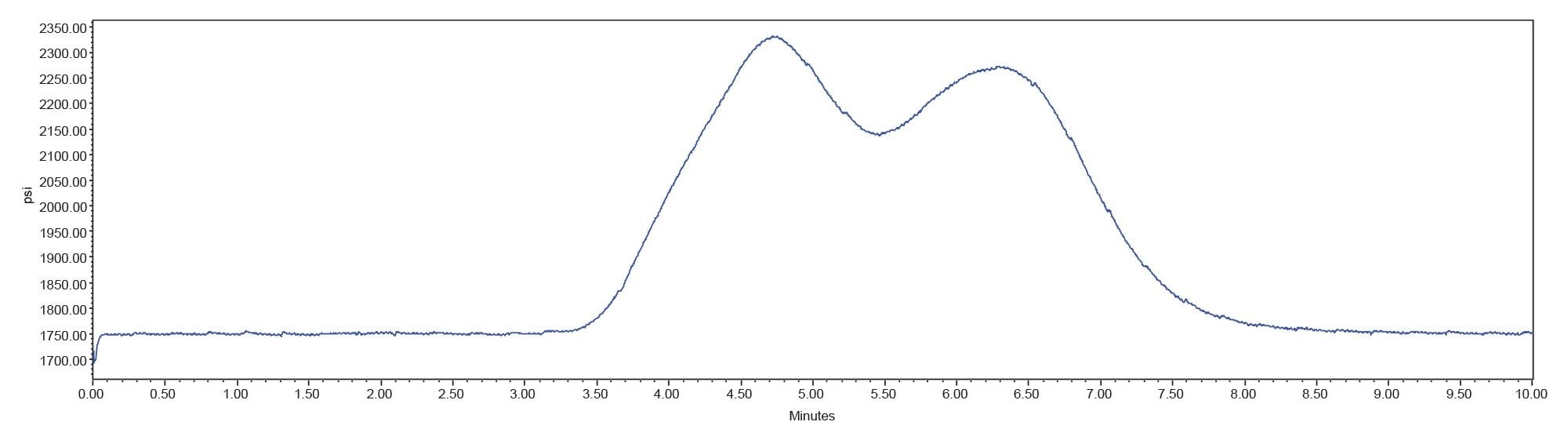
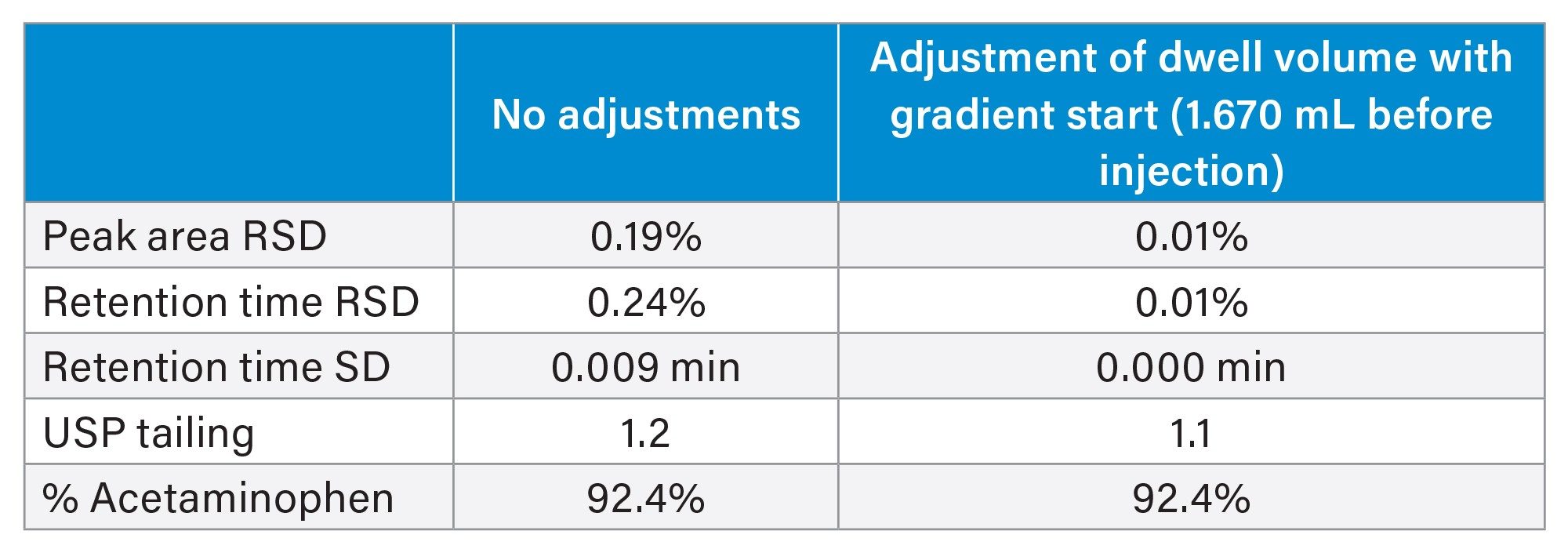
The pressure plot (Figure 5) shows that with a gradient start of 1.67 mL before injection, the system pressure returned to initial levels after the gradient was applied and held steady before the next injection was made.
The chromatograms produced using the gradient start feature have stable baselines at the start of the run (Figure 6). Since acetaminophen elutes during the isocratic portion of the method, its retention time was not affected by modifying the gradient start time. The retention time of the peak produced from the gradient wash step shifted as it elutes in the gradient portion of the method and therefore is impacted by the gradient start adjustment to the method.
The results obtained using the gradient start feature (Table 5) show improved retention time precision when compared against the results obtained without dwell volume compensation. The USP system suitability criteria were met. Results for peak area precision, tailing, and % acetaminophen are comparable to those obtained without dwell volume compensation.
Conclusion
An effective re-equilibration period for gradient HPLC methods includes both the volume for the composition change set by the instrument method to reach the column – the dwell volume - and a period where the system and column are held at initial conditions until it is stable enough for the next injection. Failure to compensate for dwell volume during re-equilibration may have an impact on chromatographic results.
In this study, the Alliance iS PDA System met the system suitability criteria for the USP Acetaminophen Tablets Assay. However, the method contained a re-equilibration volume that was lower than recommended. To assess the impact of increasing the re-equilibration, the dwell volume of the system was adjusted using the Next Injection Delay and Gradient Start tools available through the Empower Chromatography Data System and Alliance iS instrument method. The tools allowed for adjustment of dwell volume without changing the gradient table of the method. The results demonstrated adequate re-equilibration of the column and system with improved baseline stability and retention time precision.
References
- Dolan JW. How Much Is Enough. LC-GC North America. 2003 Oct;21(10):968–962.
- United States Pharmacopeia (2023) USP Monographs Acetaminophen Tablets, USP-NF. DOI: https://doi.org/10.31003/USPNF_M200_05_01.
- Stoll DR, Seidl C. Column Re-equilibration Following Gradient Elution: How Long is Long Enough? Part I: Reversed-phase and HILIC Separations of Small Molecules. LC-GC North America. 2019 Nov;37(11):790–795.
- Waters Columns Calculator Version 2.0.
Featured Products
720008768, April 2025