
In this application note, The ionKey/MS System, a novel microfluidics-MS platform, was assessed for the micro-LC analysis of pesticides in food samples. Microfluidic technology offers the capability to integrate several fluidic and instrumental components onto a single device. The rugged and easy-to-use ionKey/MS System greatly facilitates the utilization of micro-LC in high-throughput food safety laboratories. The iKey enables highly reproducible LC separation with comparable resolution to analytical scale LC-MS analysis. This was demonstrated in both peak area reproducibility and retention times for pesticides in a variety of matrices. An average of 8x improvement in sensitivity over 2.1 mm high-flow chromatography can help food and beverage laboratories meet the increasing demands of international regulatory agencies. Low flow rates of 2.3 μL/min allow for 10x savings in solvent consumption and costly hazardous waste disposal charges.
Microfluidic technology offers the capability to integrate several fluidic and instrumental components onto a single device. The process of microfluidic integration has several advantages for the micro-LC user. First, the micro-LC fittings either between the column and ESI source, or between the column and injection valve, must be precisely fitted. Even micrometers of difference between the transfer line and the column can equate to several µLs of void volume, and at µL/min flow rates can result in large deviations in retention time or peak width due to dispersion. The ability to micromachine and integrate the post-column connections on a planar microfluidic device offers the simplicity of never having to replace or change post-column lines or ESI tips. Secondly, the clamp-on microfluidic fittings make it easy to replace the microfluidic iKey Separation Device in a matter of seconds. This allows for trouble-free system maintenance, but it can also facilitate the process of method development with different column chemistries. In addition, the integrated heating elements, memory, and ESI tips require minimal programming, providing control of LC-gradients and ESI spray in a customized environment.
Operating at the micro-LC scale provides a number of advantages for minimizing laboratory solvent consumption. For pesticide screening applications, the ionKey/MS System utilizes a scaled down flow rate of 2.3 µL/min. This substantially lower rate of solvent consumption and consequential reduction of hazardous waste removal can result in significant cost savings to laboratories.
In addition to the reduction in solvent consumption, there are also significant improvements in sensitivity for many analytes. In this application note, a mixture of pesticides was spiked into food matrices of varying levels of complexity. Initial work was undertaken to compare the iKey Separation Device with a conventional 2.1 mm I.D. ACQUITY UPLC Column. For the 50 pesticides individually assessed, the sensitivity was improved by an average of 8x over the 2.1 mm format. The ionKey/MS System was further tested for robustness and performance with a range of matrices.
|
LC system: |
ACQUITY UPLC M-Class System |
|
Separation device: |
iKey BEH C18 Separation Device, 130Å, 1.7 μm, 150 μm x 100 mm (p/n 186007258) |
|
iKey temp.: |
45 °C |
|
Injection volume: |
5 μL |
|
Flow rate: |
2.3 μL/min |
|
Mobile phase A: |
Water with 10 mM ammonium acetate, pH 5.0 |
|
Mobile phase B: |
Methanol with 10 mM ammonium acetate, pH 5.0 |
|
Weak needle wash: |
Water |
|
Strong needle wash: |
Acetonitrile |
|
Seal wash: |
90:10 Water:acetonitrile |
|
Time (min) |
Flow (μL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
2.3 |
98.0 |
2.0 |
initial |
|
12.25 |
2.3 |
1.0 |
99.0 |
6 |
|
13.25 |
2.3 |
98.0 |
2.0 |
6 |
|
20.00 |
2.3 |
98.0 |
2.0 |
6 |
|
MS system: |
Xevo TQ-S |
|
Acquisition mode: |
MRM |
|
Ionization mode: |
ESI + |
|
Capillary voltage: |
4.0 kV |
|
Source temp.: |
120 °C |
|
Cone voltage: |
Variable |
|
Dwell time: |
0.003 to 0.01 s |
A standard QuEChERS AOAC (2007.01) extraction protocol was performed using the DisQuE Quechers, 900 mg MgSO4 & 150 mg PSA, 15 mL Tube (p/n 186004833), first by homogenizing the commodity in a blender, followed by weighing 15 g of the commodity into a vial. Next, 15 mL of extraction buffer consisting of acetonitrile with 1% acetic acid was added to the commodity along with pouch 1 of the DisQuE salt mixture. Vials were shaken for approximately 1 min and centrifuged at 1500 rcf for 1 min. The supernatant was removed and filtered through a 0.2 µm PFTE filter prior to dry down and reconstitution in mobile phase. Standards with 200 pesticides were constituted in extracted food matrix at levels ranging from 1 ppt to 10 ppb with 11 intervals. Matrix blanks from the extracted food matrix were collected along with solvent blanks. These blanks were used to identify the existing presence of pesticides in the commodity.
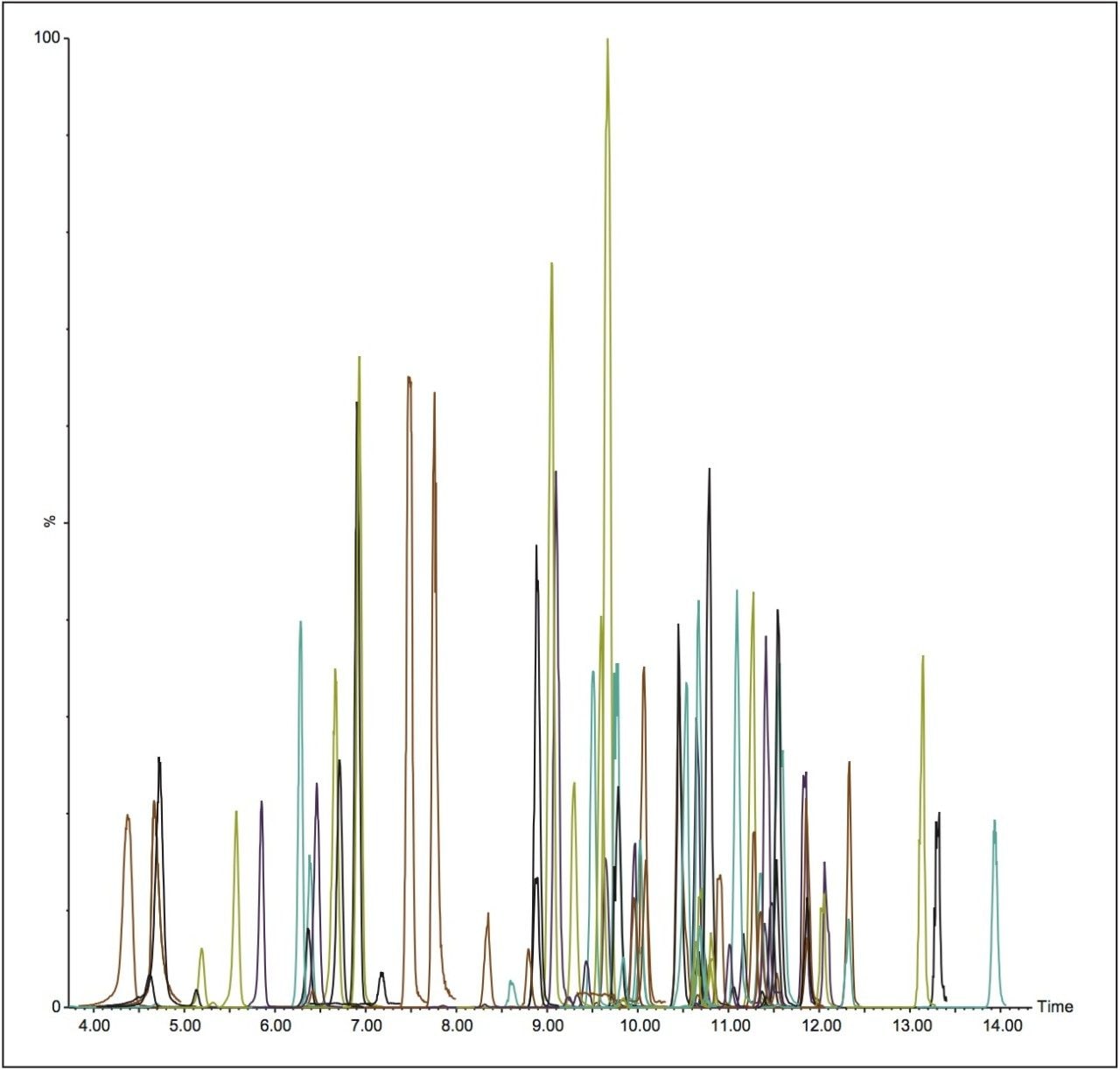
In order to determine the feasibility of the ionKey/MS System for pesticide residue analysis, a method that incorporated 360 MRM transitions was employed, even though not all of the pesticide standards for the method were spiked. This enabled assessment of the data quality that would be obtained with a typical multi-residue pesticide method. Figure 1 illustrates a total ion chromatograms (TIC) containing 99 pesticides in onion matrix separated and detected using the ionKey/MS System. Several matrices were analyzed with this system including infant formula, summer squash, onion, and tomato.
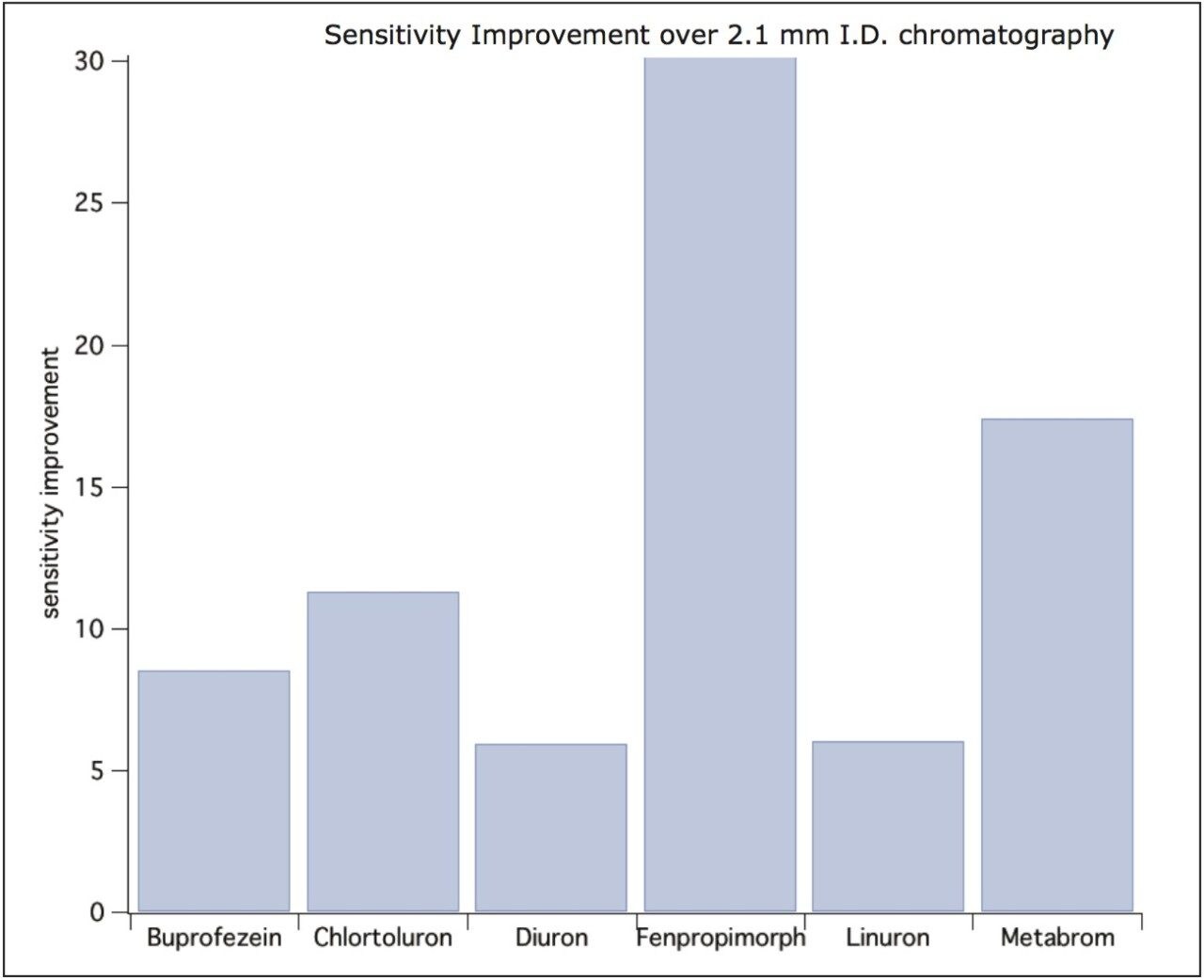
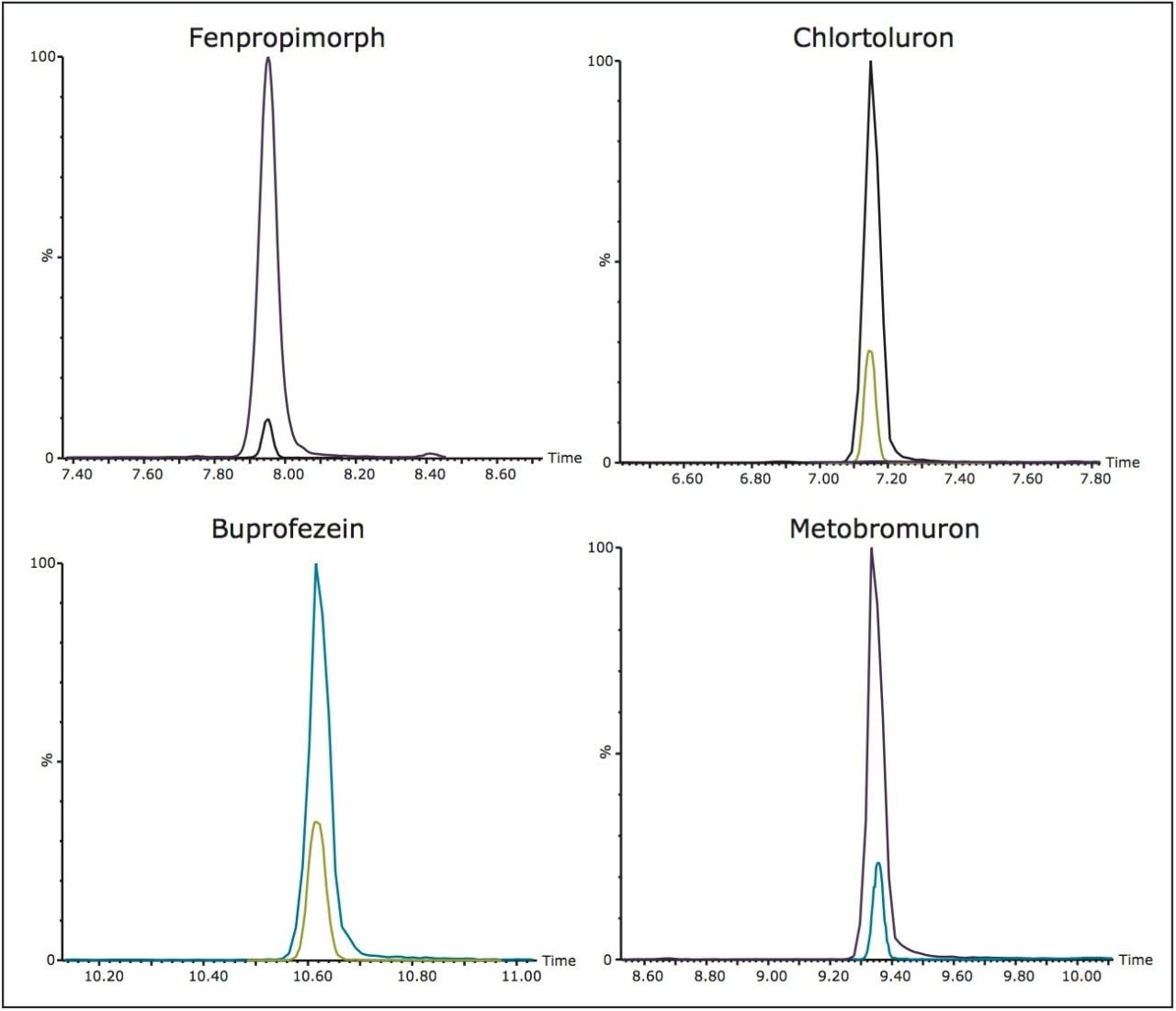
Peak widths at half height were similar to the 2.1 mm separations ranging from 3 to 6 s. However, the sensitivity of the ionKey/MS System was on average 8x greater compared to regular analytical columns. The improvement in sensitivity is due to the improved electrospray at low flow and the reduced dilution that occurs within the iKey Separation Device. These two factors help increase the number of ions entering the Xevo TQ-S, resulting in improved sensitivity. The sensitivity enhancement observed in the ionKey/MS System is illustrated in Figure 2 and Figure 3. Figure 2 shows a series of six pesticides in infant formula displaying different improvements in sensitivity over 2.1 mm I.D. chromatography. The differences in ionization efficiency are based on molecular structure, hydrophobicity, and acid-base functionality. Pesticides that have non-polar functionalities are driven towards the surface of the droplet. If pesticides reside at the surface of a droplet for longer periods of time they are more likely to enter the gas phase as an ion. The greatest improvement was observed with fenpropimorph with a 25x improvement seen in infant formula. In Figure 3, MRM chromatograms of four different pesticides are displayed with the iKey peaks Separation Device overlaid with the 2.1 mm I.D. peaks. As can be seen from these chromatograms, the ionKey/MS System offers improved signal-to-noise ratio over the 2.1 mm I.D. chromatography.
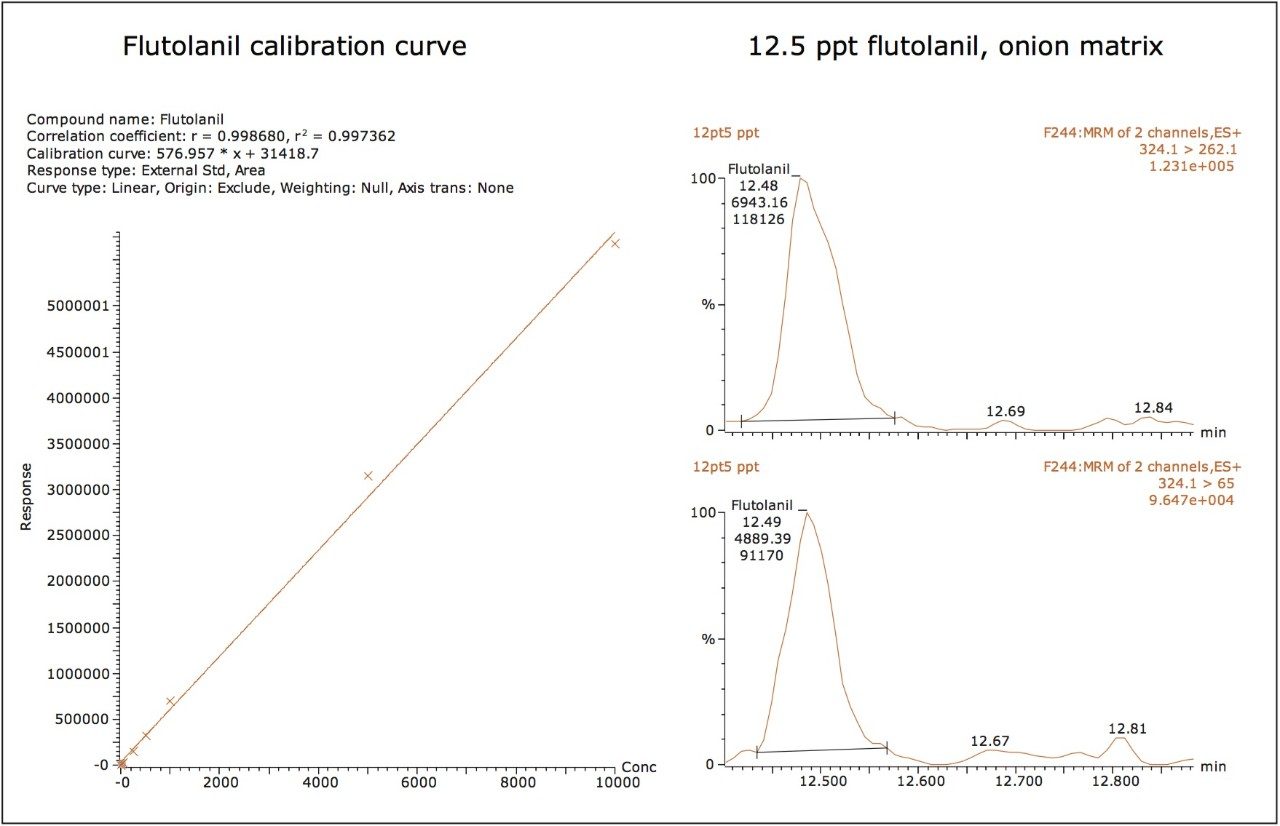
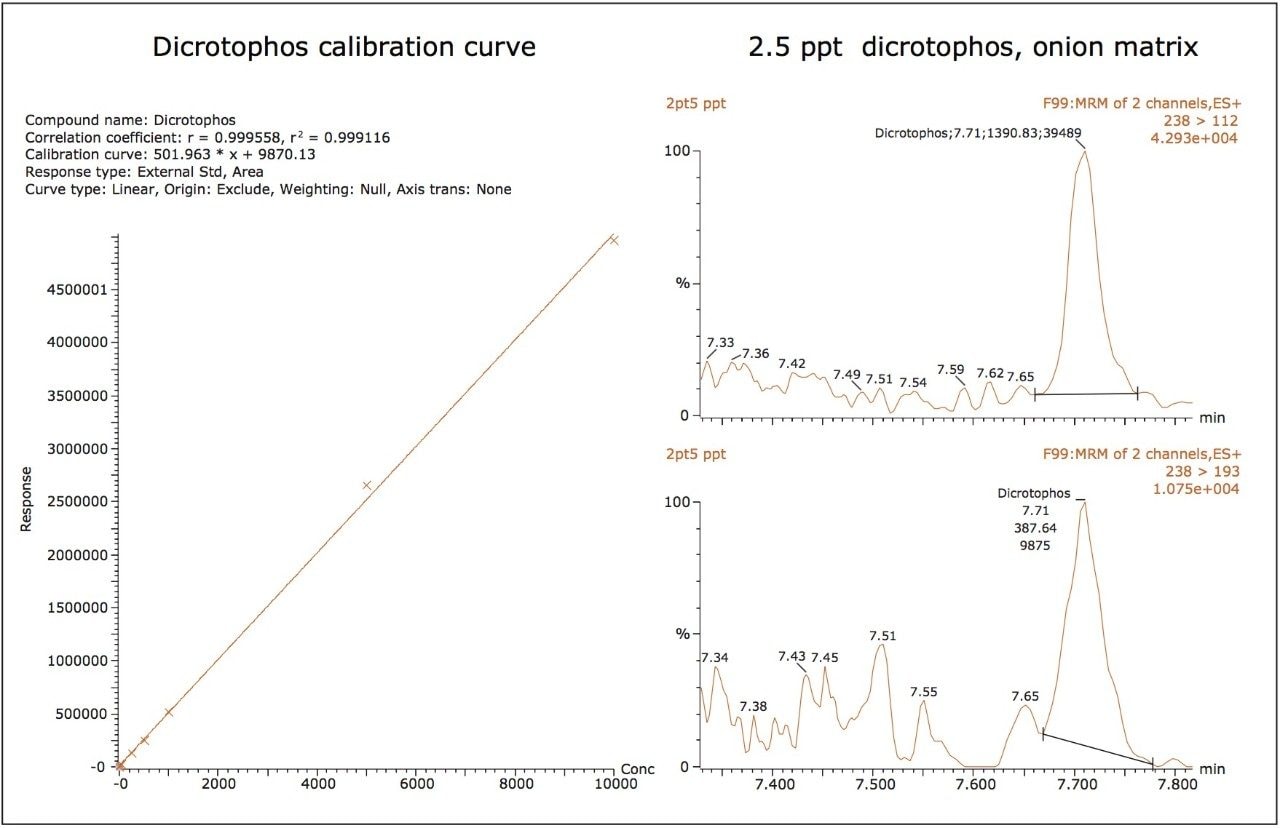
The dynamic range of the ionKey/MS System was also shown to be greater than 3 orders of magnitude for the majority of compounds investigated here. Figures 4A and 4B show the linearity of the calibration curves for dicrotophos and flutolanil, which demonstrated R2 values >0.99. The MRM chromatograms of both the primary and secondary ions for both dicrotophos and flutolanil at their LOQs (2.5 and 12.5 ng/L, respectively) are also shown. The calculated peak-to-peak, signal-to-noise (S/N) for the dicrotophos primary ion was 12 and 35 for flutolanil.
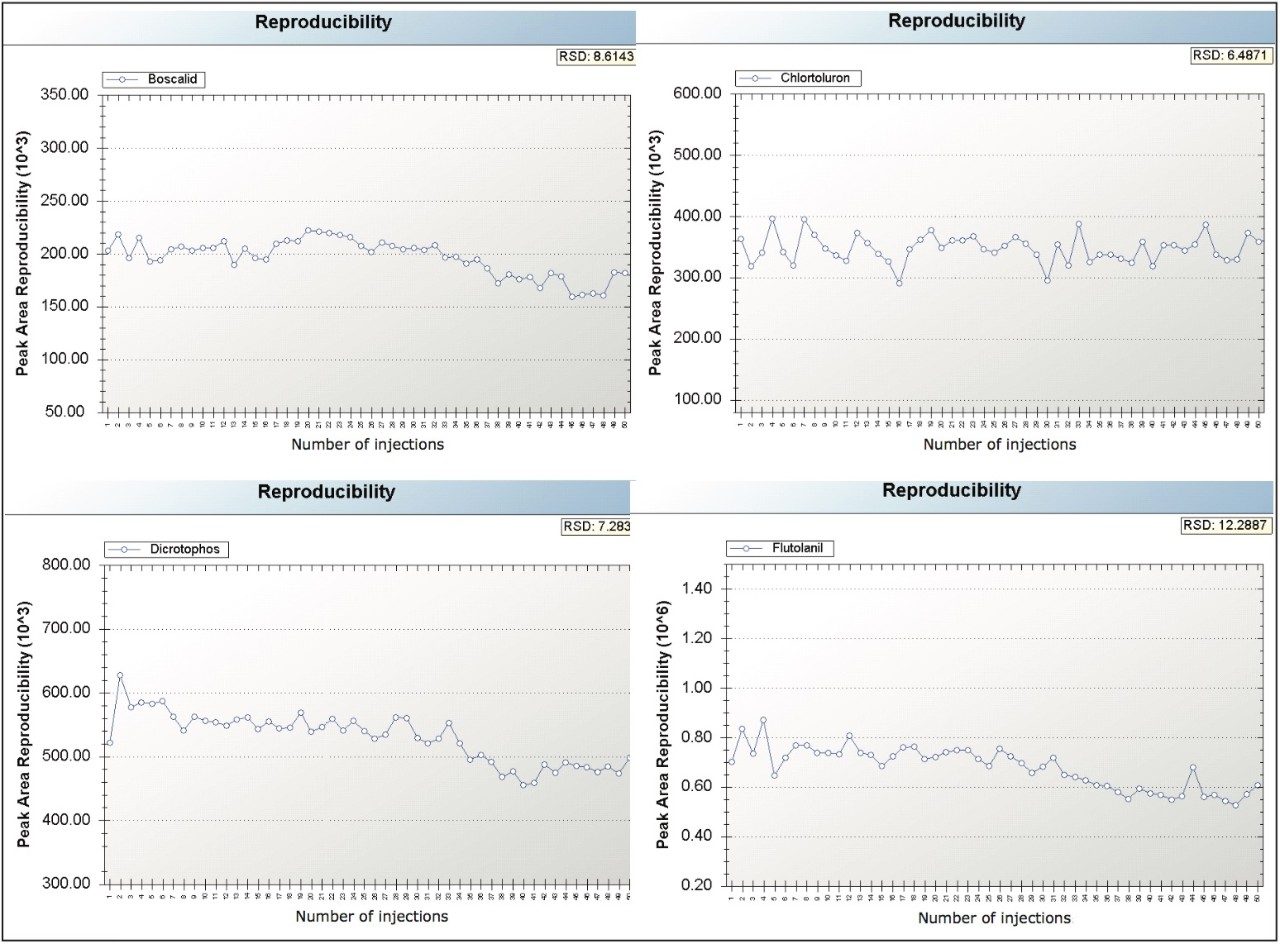
Microfabrication of fluidic connections allows for precise connections with limited to no dead volume, and limited variation from chip to chip. Using TrendPlot we plotted peak area and retention time reproducibility, shown in Figures 5 and 6. We found peak area and retention time reproducibility to be below 13% RSD and 1% RSD, respectively, for boscalid, flutolanil, chlortoluron, and dicrotophos at 1 µg/L in tomato matrix. In addition to investigating peak area and retention time reproducibility, we also investigated ruggedness of use.

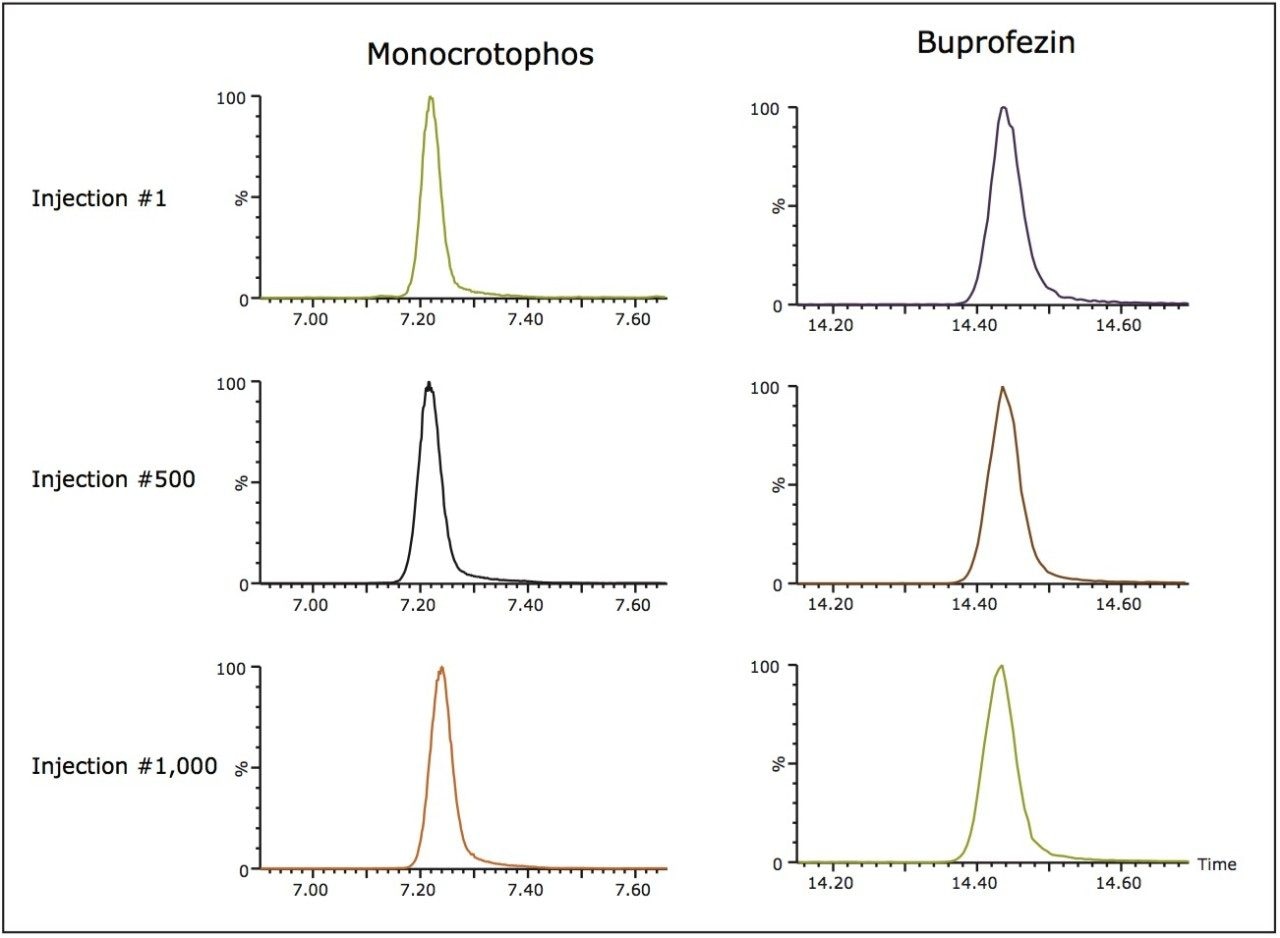
After 1,000 injections of infant formula extract we found negligible increases in pressure and reproducible peak retention times. Figure 7 illustrates peak retention reproducibility of buprofezein and monocrotophos at injections 1, 500, and 1,000.
The ionKey/MS System is a novel microfluidics-MS platform for rugged and easy-to-use micro-LC analysis of pesticides in food samples. The iKey Separation Device enables highly reproducible LC separations with comparable resolution to analytical scale LC-MS analysis. This was demonstrated in both peak area reproducibility and retention times for pesticides in a variety of matrices.
An average of 8x improvement in sensitivity over 2.1 mm high-flow chromatography can help food and beverage laboratories meet the increasing demands of international regulatory agencies.
Low flow rates of 2.3 µL/min allow for 10x savings in solvent consumption and costly hazardous waste disposal charges to improve a laboratory’s bottom line.
720004976, January 2016