
Knowledge of the efficacy of the individual enantiomers of pesticides is of great interest to agrochemical companies. The benefits of using these products can be realized only when the optimal application rates are determined and when the appropriate metabolic and toxicological evaluations are performed. However, the ability to separate the enantiomers is required in order to study the effects of each individual isomer.
Experiments concerning the steroeselective behavior of the individual pure isomers of chiral pesticides depend on the development of the necessary preparative separation technology that enables them to be readily separated and isolated as single stereoisomers.
Although traditionally dominated by liquid chromatography (LC), there is an increasing trend toward using supercritical fluid chromatography (SFC) to replace normal phase LC for chiral purifications from the semi-preparative scale up to the kilogram scale.
In this application note, we present the chiral resolution of metalaxyl using the ACQUITY UPC2 System, followed by scaling to a semi-preparative purification using the Investigator SFC System. this workflow is ideal for agrochemical companies facing escalating pressure to discover, develop, and release new products into the marketplace while adhering to strict deadlines and budget constraints.
Knowledge of the efficacy of the individual enantiomers of pesticides is of great interest to agrochemical companies. The benefits of using these products can be realized only when the optimal application rates are determined and when the appropriate metabolic and toxicological evaluations are performed. However, the ability to separate the enantiomers is required in order to study the effects of each individual isomer. Experiments concerning the steroeselective behavior of the individual pure isomers of chiral pesticides depend on the development of the necessary preparative separation technology that enables them to be readily separated and isolated as single stereoisomers.
Although traditionally dominated by liquid chromatography (LC), there is an increasing trend toward using supercritical fluid chromatography (SFC) to replace normal phase LC for chiral purifications from the semi-preparative scale up to the kilogram scale. Due to the higher diffusivity and lower viscosity of supercritical fluid, SFC often provides a three- to eight-fold faster separation than normal phase HPLC, resulting in a measurable increase in productivity. Compared to normal phase LC, SFC also offers unique selectivity, less organic solvent consumption and related waste removal, smaller collection volume, and faster post-purification dry-down time. Generally speaking, SFC is as a more cost-effective and environmentally friendly preparative chromatographic technique.
Development of SFC purification begins with analytical method development to identify optimal stationary phases and mobile phases. This step can be completed rapidly using the Waters ACQUITY UPC2 System, and the resulting method can then be scaled up for preparative purifications using the Investigator SFC System.
Combined with the technical advantages of SFC, this workflow is ideal for agrochemical companies facing escalating pressure to discover, develop, and release new products into the marketplace while adhering to strict deadlines and budget constraints.
Metalaxyl, a phenylamide fungicide, with one chiral center in its chemical structure (Figure 1), owes its fungicidal activity predominantly to the R enantiomer.1 In this application note, we present the chiral resolution of metalaxyl using the ACQUITY UPC2 System, followed by scaling to a semi-preparative purification using the Investigator SFC System.
|
Analytical system: |
ACQUITY UPC2 |
|
Column: |
Chiralpak IA-3, 4.6 x 150 mm, 3 μm |
|
Flow rate: |
3.5 mL/min |
|
Mobile phase composition: |
4% 2-propanol in CO2 |
|
ABPR: |
2000 psi/138 bar |
|
Column temp.: |
55 °C |
|
Detector: |
ACQUITY UPLC PDA |
|
UV detection: |
215 nm |
|
Prep system: |
Investigator SFC |
|
Column: |
Chiralpak IA, 10 x 150 mm, 5 μm |
|
Flow rate: |
12 mL/min |
|
Mobile phase composition: |
8% 2-propanol in CO2 |
|
ABPR: |
1600 psi/110 bar |
|
Column temp.: |
30 °C |
|
Detector: |
2998 PDA |
|
UV detection: |
215 nm |
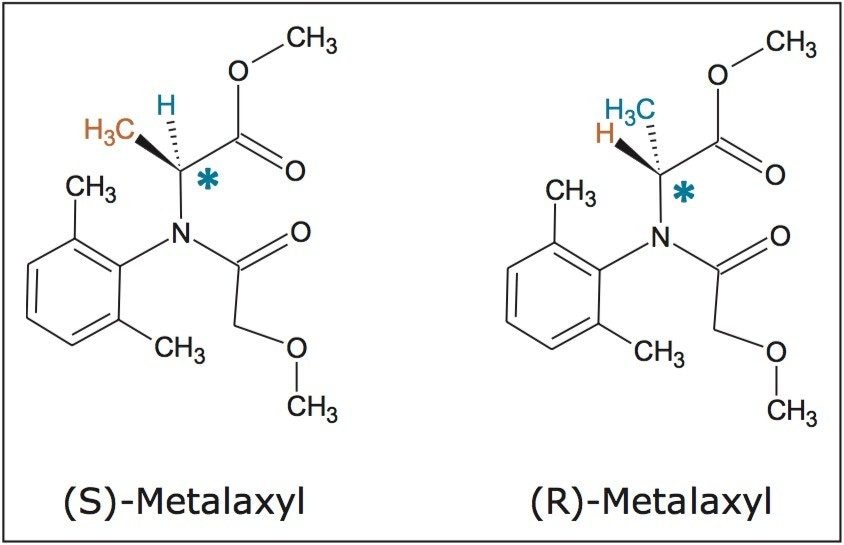
Analytical experiments were performed on an ACQUITY UPC2 System with PDA detection at 215 nm. Semi-prep experiments were performed on the Investigator SFC System. The system consists of the following: Fluid Delivery Module (FDM), Automated Back Pressure Regulator (ABPR), Alias Autosampler, 10-port Analytical-2-Prep Column Oven, 2998 PDA Detector, make-up pump, and six-position Fraction Collection Module. The system is controlled by ChromScope Software.
A racemic (R, S)-metalaxyl standard was obtained from Sigma-Aldrich (St. Louis, MO). The sample was dissolved in methanol at 1 mg/mL and 20 mg/mL, for analytical and preparative injections, respectively.
A Chiralpak IA-3 (4.6 x 150 mm, 3 μm) was used for the analytical separation.
SFC purification often starts with gradient screening of multiple chiral stationary phases and mobile phases. After the column and solvent conditions that provide the highest enantioselectivity for the analyte are determined, the gradient method is converted to an isocratic separation. The ACQUITY UPC2 System has multi-column switching capabilities and a choice of four co-solvents. The method development process can be completed rapidly due to the shorter analysis times that are possible using this technique. The IA-3 column yielded the highest resolution between the enantiomeric pair (Figure 2). It was, therefore, chosen for the remaining experiments.
Isocratic conditions are necessary for employing stacked injections in preparative SFC for high productivity. Resolution and run time, the two competing factors for overall productivity, should be carefully considered when developing an appropriate isocratic method. Typically, a higher percentage of co-solvent leads to a shorter run time but also to reduced resolution. Resolution is also affected in preparative chromatography by larger mass/volume injections. A successful isocratic method often involves a compromise between run time and resolution which is determined experimentally. In this case, an isocratic eluent containing 4% co-solvent appeared to be a suitable choice on the analytical scale.

The major differences between the analytical and semi-prep run parameters are due to the different particle sizes used in the columns (3 µm versus 5 µm), and to the differences in system volumes between the UPC2 and the Investigator systems. The run parameters were adjusted to account for these differences to give favorable resolution, loading and run times.
A loading study was then conducted on a 10-mm I.D. x 150 mm IA column to determine the maximum loading for preparative runs. The results are shown in Figure 3. The injection volumes used were 20 μL, 40 μL, and 80 μL. At 80 μL, the metalaxyl enantiomers were still separated. The maximum effective loading was 80 μL or 1.60 mg in mass per injection.
Stacked injection is an effective means to improve productivity without compromising chromatographic efficiency. Figure 4 shows the SFC chromatograms of (R, S)-metalaxyl (20 mg/mL) from a sequence of five stacked injections. Each injection was 80 μL of a 20 mg/mL racemic solution. A total of 8 mg (4 mg for each enantiomer) was purified in the five stacked injections over 20 minutes. The total solvent use was 20 min x 8% x 12 mL/min = ~19 mL. Performed under isocratic conditions, injections are made during the course of chromatography so that the first peak from a subsequent injection elutes off the column adjacent to the last peak of the preceding injection. Additionally, resolution is affected in preparative chromatography by larger mass/volume injections. The chromatographic space is efficiently populated by peaks from consecutive injections, resulting in noticeable savings of both time and solvents. ChromScope Software manages the injection and collection intervals such that fractions are predictably collected efficiently.

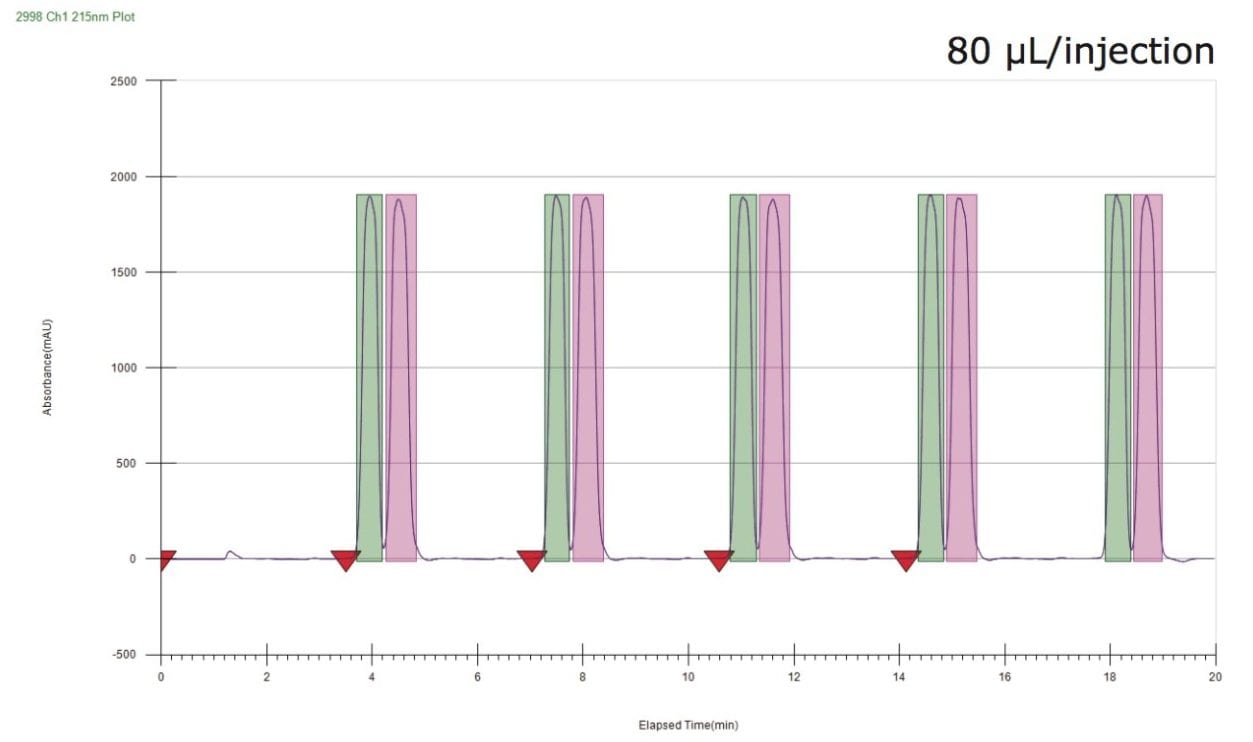
Sample purification was confirmed by analyzing the two collected fractions using the UPC2 isocratic analytical method and the resulting chromatograms are shown in Figure 5. Both fractions showed >99% purity. Absolute configurations were assigned using a commercially available standard of metalaxyl-M (the (R) isomer).
Using racemic metalaxyl as a model compound, the Investigator SFC System was shown to be a powerful tool in meeting the challenges of chiral separation and purification on a single platform. Chiral screening using the ACQUITY UPC2 System quickly determined the optimal column for the separation of R- and S-metalaxyl. An isocratic method was developed to take advantage of stacked injection purification and the method was easily scaled up following logical principles concerning the preservation of resolution, maintaining reasonable run times and the maximization of loading. Stacked injections using the SFC technique allowed for the purification of 24 mg/hour, yielding high purity fractions of the metalxyl enantiomers in a smaller volume of organic solvent than would be traditionally used in LC purification. Longer sequences of a larger number of stacked injections could also be employed to give more purified product. The complete workflow of SFC in chiral screening, method development, scale-up, and efficient small scale prep using stacked injections has been successfully demonstrated using UPC2 in conjunction with the use of the Investigator SFC System, meeting the increasing demand for fast chiral resolutions. Reduced solvent consumption coupled with reduced dry-down time has a positive impact on the laboratory by increasing the throughput while reducing the cost of processing each sample.2
720005312, February 2015