For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the analytically sensitive and precise measurement of adrenal steroids from limited-volume clinical research serum samples.
The data presented indicates the ACQUITY UPLC I-Class (FTN) with Xevo TQD is capable of meeting the analytical precision necessary for measurement of physiologically-relevant concentrations of serum 17-OHP for clinical research. The narrow diameter tubing of the ACQUITY UPLC I-Class System creates a low-dispersion system allowing for greater LC peak capacity and precision than achieved before with the ACQUITY UPLC System . The inclusion of active pre-column heating of mobile phase brings further advantages in terms of column efficiency, improving reproducibility of peak area integration. Precision at low concentrations contributes to overall analytical sensitivity (Table 2). With further optimization of LC methods to improve detection of A4, this configuration can be used in laboratories with low to moderate detector sensitivity needs.

The challenge of serum 17-hydroxyprogesterone, cortisol, and androstenedione measurement is made routine with UPLC-MS/MS for clinical research.
Precise measurement of low concentrations of steroids in limited-volume complex matrices such as serum or plasma poses an analytical challenge which previously called for a high performance mass detector such as the Xevo TQ-MS. This work evaluates the utility of combining improved chromatographic resolution, column efficiency, and reproducibility afforded by the ACQUITY UPLC I-Class System (FTN) with the entrylevel Xevo TQD.
Analytes were extracted from limited-volume serum samples using a non-derivatized, single liquid-liquid-extraction procedure. Analytically sensitive quantification was achieved by the superior resolution of the ACQUITY UPLC I-Class System (FTN) in combination with the robust simplicity of the Xevo TQD.
Deuterated internal standard-spiked serum (50 μL) was mixed with methyl-tert-butyl-ether (1 mL). Steroids were extracted into the organic layer which was transferred to a maximum recovery vial, evaporated to dryness and reconstituted in 50 μL of 45% (v/v) aqueous methanol. Chromatographic separation of the injected 20-μL sample was achieved using an ACQUITY UPLC HSS T3, 2.1 x 50 mm Column (p/n 186003538) fitted with a chemistry-matched VanGuard 2.1 x 5 mm Pre-column (p/n 186003976). A three-stage gradient separation over 2.5 minutes enabled the resolution of the compounds of interest, in addition to a number of isobaric steroid intermediates. The injectioninjection time was 5.0 minutes to permit adequate LC column washing and re-equilibration.
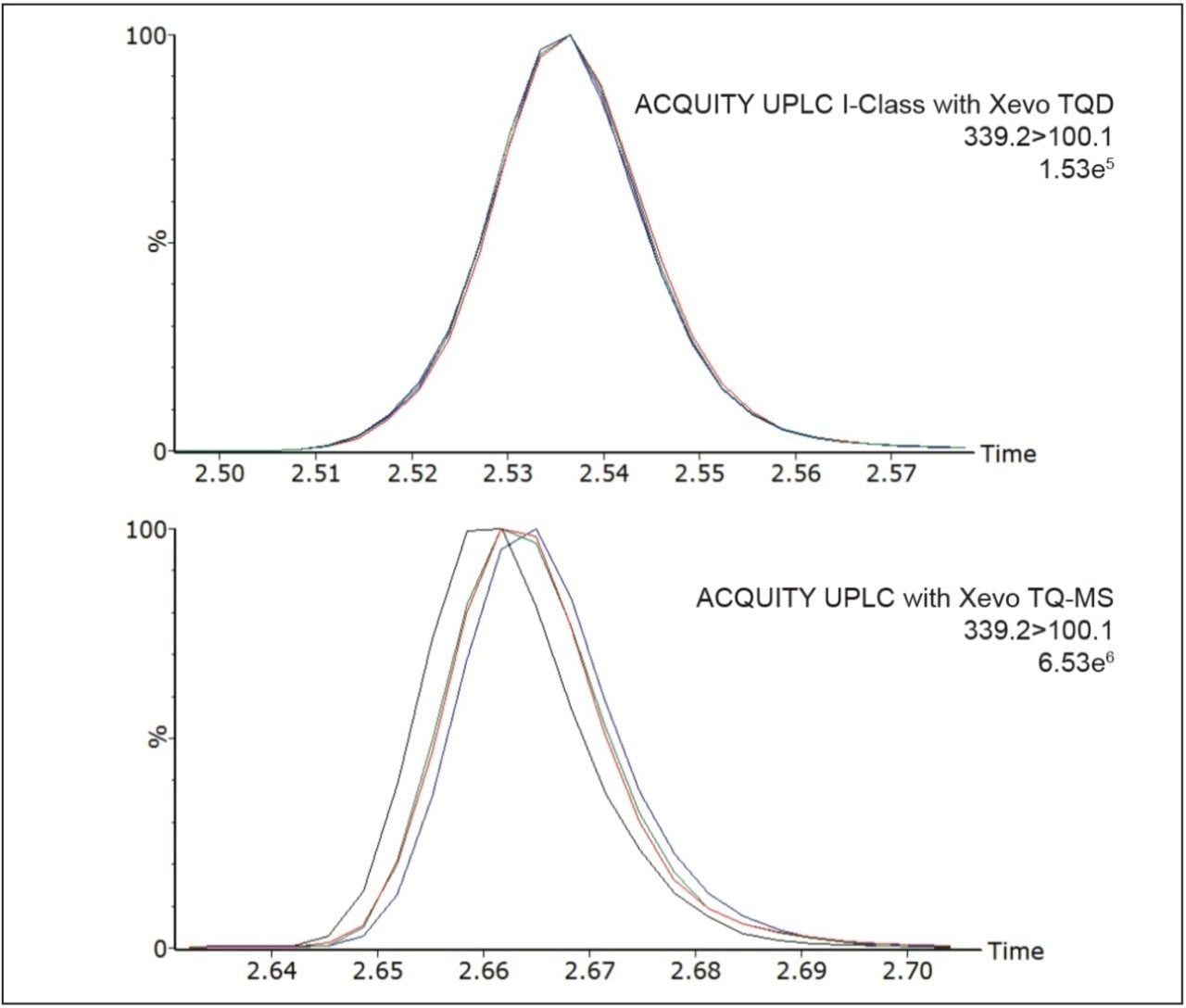
The performance of the ACQUITY UPLC I-Class System (FTN) with Xevo TQD was evaluated in comparison with the ACQUITY UPLC System with Xevo TQ-MS. Analysis of multiple levels of inhouse prepared stripped-serum quality controls (QC) highlighted the superior precision achievable with the ACQUITY UPLC I-Class (FTN) with Xevo TQD (n = 5, over 5 occasions, 2.5 – 75 ng/mL for A4 and to 250 ng/mL for 17-OHP and cortisol). Calculation of 17-OHP internal standard chromatogram peak widths using TargetLynx Application Manager processing confirmed the improved reproducibility of peak widths using the ACQUITY UPLC I-Class (FTN). Example overlaid chromatograms of 17-OHP internal standard from extracted QCs are shown in Figure 2.
Replicate analyses of stripped-serum calibrators enabled like-for-like comparison of the analytical sensitivity of the two systems. The peak-to-peak signal-to-noise ratio (S/N) of calibrator 1 (0.5 ng/mL) was comparable between the two systems for 17-OHP. Further method optimization is required to match the analytical sensitivity of the two systems for cortisol and A4 (Table 2).
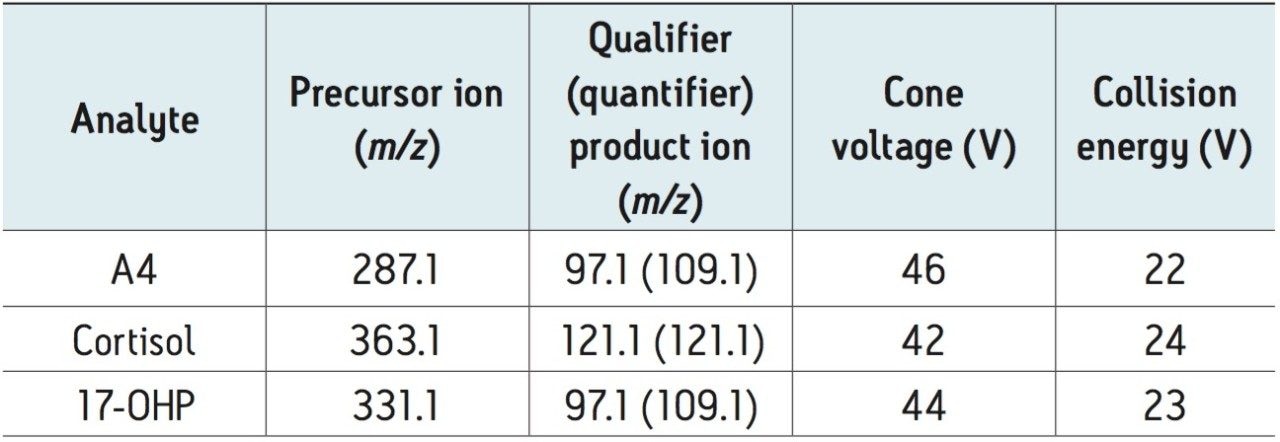
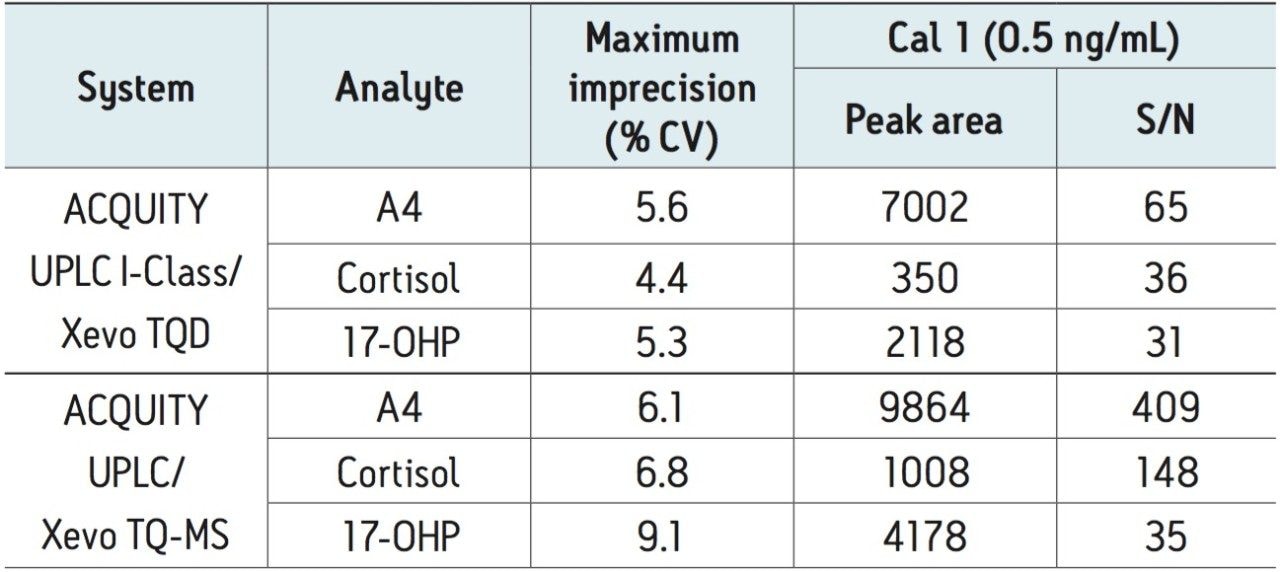
The data presented indicates the ACQUITY UPLC I-Class (FTN) with Xevo TQD is capable of meeting the analytical precision necessary for measurement of physiologically-relevant concentrations of serum 17-OHP for clinical research.
The narrow diameter tubing of the ACQUITY UPLC I-Class System creates a low-dispersion system allowing for greater LC peak capacity and precision than achieved before with the ACQUITY UPLC System (Figure 2). The inclusion of active pre-column heating of mobile phase brings further advantages in terms of column efficiency, improving reproducibility of peak area integration. Precision at low concentrations contributes to overall analytical sensitivity (Table 2). With further optimization of LC methods to improve detection of A4, this configuration can be used in laboratories with low to moderate detector sensitivity needs.
720005273, January 2015