Taking Advantage of 12k psi Pressure Capabilities for Modernizing USP Methods on the Alliance™ iS HPLC System
Amanda B. Dlugasch, Paula Hong
Waters Corporation, United States
Published on August 19, 2025
Abstract
Pharmaceutical companies frequently use compendial high performance liquid chromatography (HPLC) methods for the analysis of raw materials and finished products. Modernization of older HPLC methods, which can include scaling the method on newer column or liquid chromatography (LC) technologies, can help to ensure product quality and safety while taking advantage of newer technologies and aligning with green chemistry.1 Specifically, compendial methods that are frequently used can be scaled to smaller particle size columns to increase throughput while maintaining the analytical performance of the method. The United States Pharmacopeia (USP) compendial methods are often written for HPLC columns which use >3 µm particles resulting in long run times at higher flow rates. Although the USP methods prescribe specific conditions, isocratic and gradient methods are capable of being scaled to columns which have a smaller particle size and shorter column length. This results in faster run times and lower solvent consumption. The USP has outlined in the General Chapter <621>2 acceptable column and method adjustments to allow scaling of isocratic and gradient methods to provide the same if not improved performance of a method. These allowable adjustments include scaling particle size and column dimensions to maintain the L/dp ratio, where L is the length of the column and dp is the diameter of the particle size of the column packing material, as well as adjusting the flow rate and injection volumes accordingly. Typical HPLC system pressures have lower limits than the Alliance iS HPLC System. Common HPLC systems have a system pressure limit of around 9,000 psi whereas the modernized Alliance iS HPLC System has a system pressure limit of 12,000 psi.
Benefits
- The Alliance iS HPLC System with PDA Detector provides a higher system operating pressure that allows for scaling methods to smaller particle size columns
- Improved throughput and decreased solvent consumption are obtained by scaling isocratic and gradient HPLC methods to newer column technologies with smaller particles sizes and shorter column lengths
- The USP quetiapine assay method run time was reduced by 57% and solvent consumption was reduced by 71%
- The USP quetiapine impurities gradient method was reduced by 51% and solvent consumption was reduced by 57%
Introduction
The continual development and modernization of pharmaceutical procedures help to ensure product quality and safety while taking advantage of new instruments and column technologies. Specifically, HPLC methods can be scaled to smaller particle columns to increase throughput while maintaining the analytical performance of the method. Method scaling also provides a greener method. When scaling a method, it is important to consider characteristics of the LC system operating pressure and how this might impact the chromatographic results. The LC system operating pressure may impose a physical limitation on the ability to scale some methods due to higher system pressure from the smaller particle size columns. The Alliance iS HPLC System is a modern HPLC system with a system pressure limit of 12,000 psi compared to typical HPLC system pressures with a limit of around 9,000 psi. With an increase in system pressure over other HPLC systems, the Alliance iS HPLC System allows for a greater capacity to scale a USP legacy method to smaller particle size columns since the newer technology of smaller particle size increases the overall system pressure of a method. The advantage of higher pressure also requires columns that can operate at these conditions.
In this study, the USP assay and impurity methods for quetiapine fumarate3 will be analyzed on the Alliance iS HPLC System with compendium columns and then scaled to a smaller particle size column using the Waters™ Columns Calculator. The method will be scaled to 3 mm, 2.5 µm columns to take advantage of the higher system pressures. The scaled method will then be compared to the original HPLC method to ensure no loss of chromatographic performance or quantitative analysis. The chromatographic assessment is based upon the resolution, tailing factor, %RSDs of area and retention time specified for each method in the monograph.3 The quantitative performance is assessed by the calculated active pharmaceutical ingredient (API) and the calculated impurities of an unknown sample. The scaled methods for both the assay and the impurity method will be assessed by the analytical method greenness scores (AMGS) Metric for Greener HPLC4 methods as it provides decreased run times and lower solvent consumption.
Experimental
Sample Preparation
The quetiapine fumarate standard (catalog#: 1592704), and the quetiapine system suitability standard (catalog#: 1592715) were purchased from the USP. The unknown quetiapine fumarate sample was purchased from alibaba.com.
Method Conditions
|
LC system: |
Alliance iS PDA HPLC System |
Quetiapine Assay (Isocratic) Method
All samples were diluted in mobile phase to the following concentrations: 1.0 mg/mL for the system suitability solution and 0.08 mg/mL for the standard solution and sample solution.
Method Conditions
|
Mobile phase: |
54:7:39 Methanol: Acetonitrile: Buffer |
|
Buffer: |
2.6 g/L of dibasic ammonium phosphate adjusted to pH 6.5 with phosphoric acid |
|
PDA wavelength: |
230 nm at 4.8 nm resolution |
LC Conditions
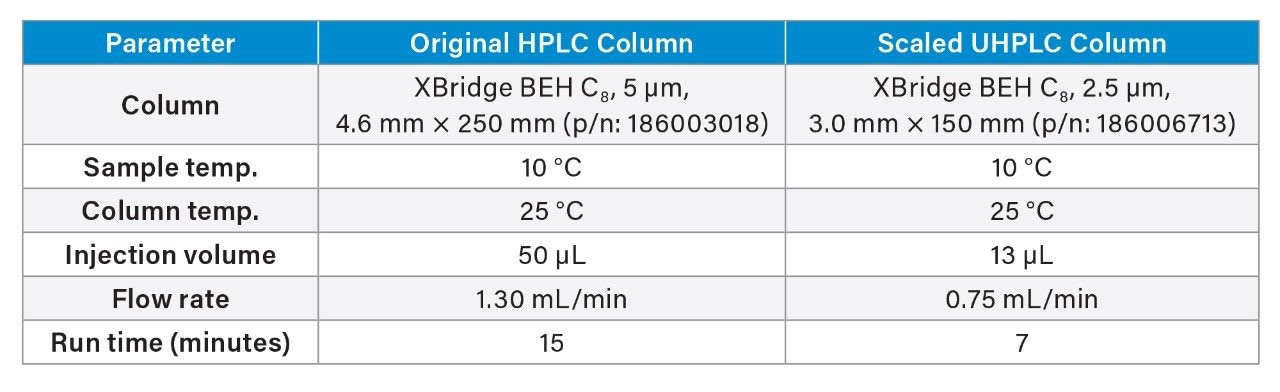
Quetiapine Impurities (Gradient) Method
The system suitability and the standard solutions were prepared in diluent (Solution A: Solution B, 86:14). The concentrations are 1.0 mg/mL for the system suitability solution and 0.001 mg/mL for the standard solution. The unknown sample solution was prepared in Solution A at a concentration of 1.0 mg/mL.
Method Conditions
|
Mobile phase: |
Solution A: 25:75 Acetonitrile: Buffer Solution B: Acetonitrile Buffer: 3.1 g/L of ammonium acetate in water. 2 mL of 25% ammonium hydroxide was added to each 1 liter of solution. The final pH is not less than (NLT) 9.2. |
|
PDA wavelength: |
250 nm at 4.8 nm resolution |
LC Conditions
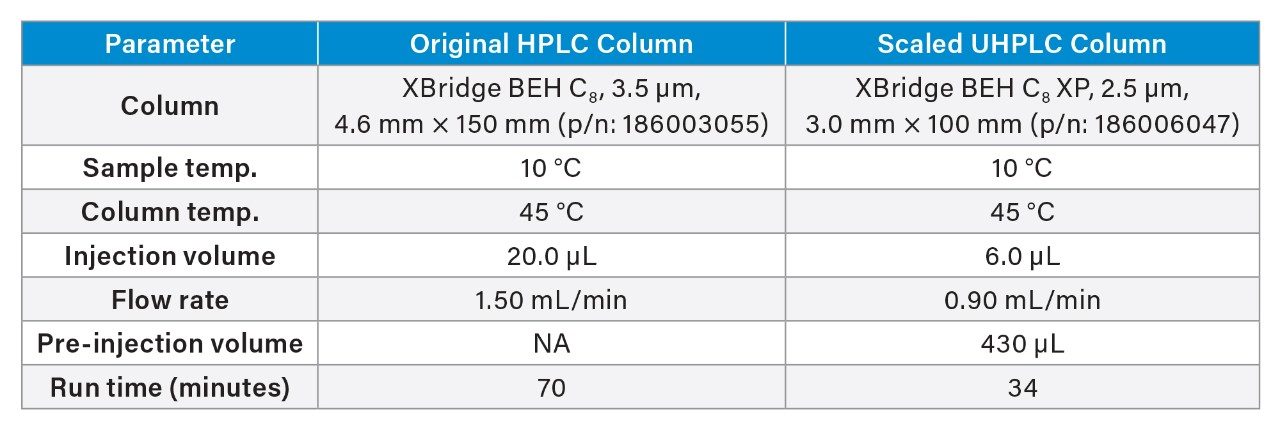
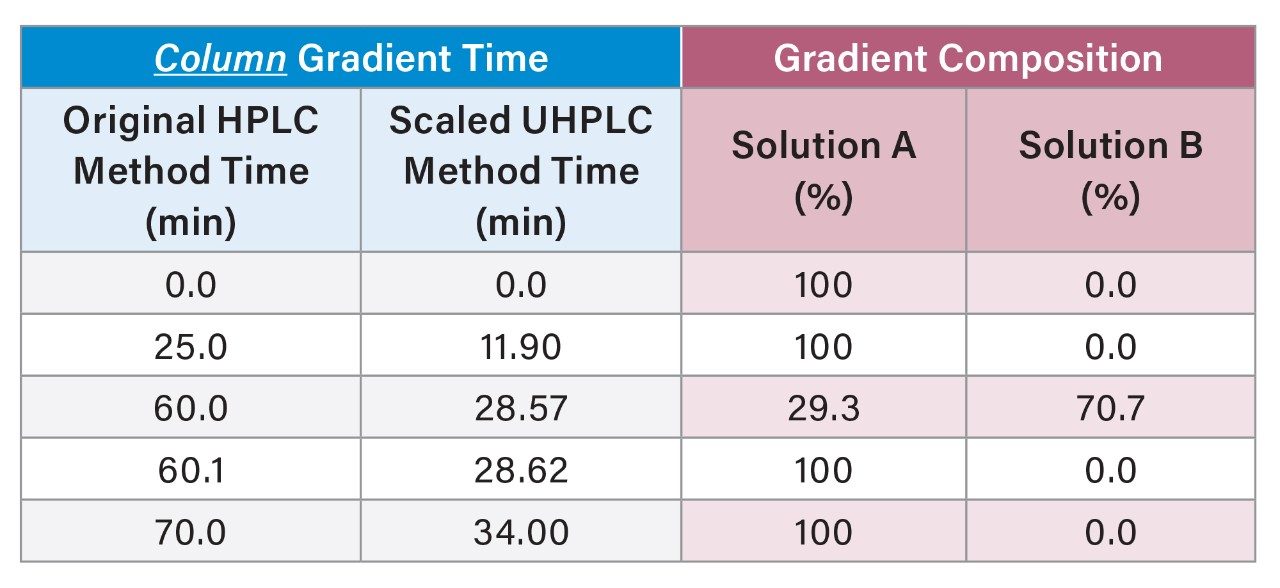
Gradient Table
Data Management
|
Data management: |
Empower Chromatography Data System (CDS) |
Results and Discussion
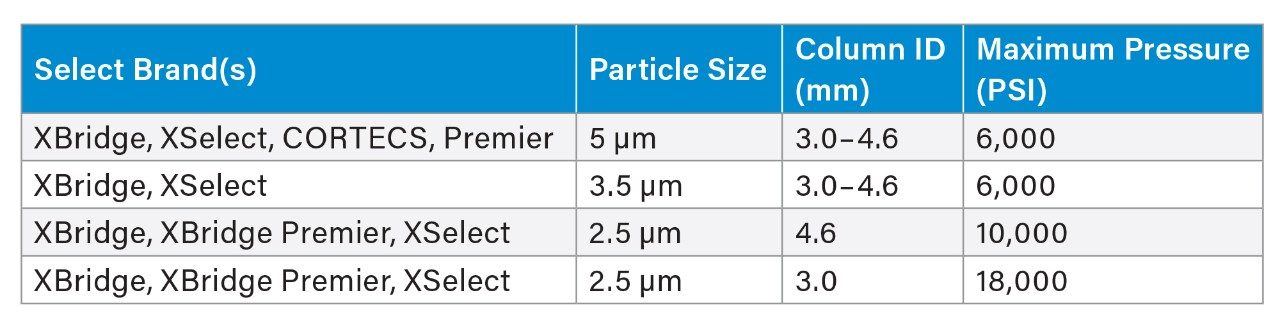
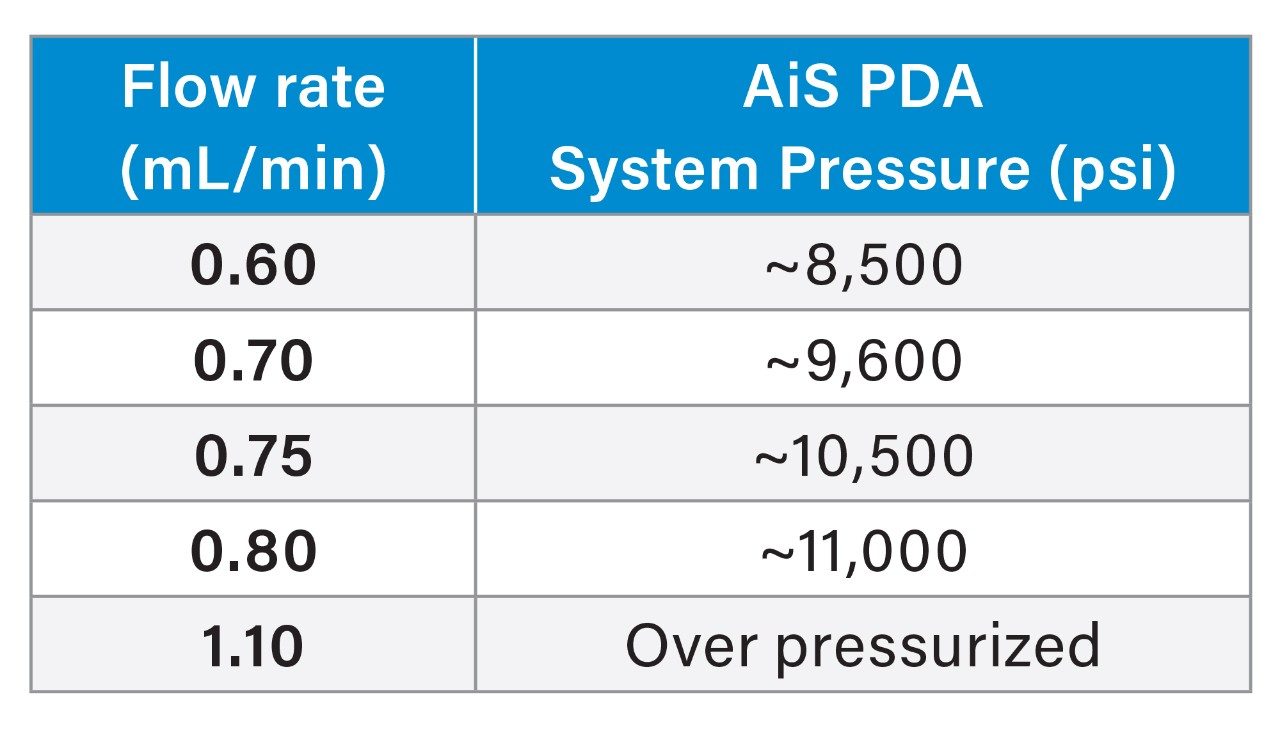
When scaling the column and method conditions using USP Chapter <621> on a single instrument, it is important to consider the operating pressure limit of the LC system. When column particle size and column dimensions are scaled to smaller particles and ID, the resulting backpressure also typically increases. Therefore, scaling some methods may not be possible due to the pressure limits of the LC system. The Alliance iS HPLC System has the highest operating system pressure and allows for more flexibility when scaling methods with an operating pressure of 12,000 psi. The Waters Columns Calculator tool can provide an estimated system pressure for a scaled method,5 in which, if necessary, certain parameters, such as the flow rate, may be altered to decrease the overall system pressure and allow for the method to be scaled successfully. When using smaller particle size columns, it is important to review the care and use manuals to determine the pressure limits of the column since different columns have different maximum pressure limits. Using columns with higher pressure limits also allows more flexibility with smaller particle sized columns and lower dimensions. Select Waters columns and general values are shown in Table 1 below.
The quetiapine assay and impurities methods were first analyzed on the Alliance iS HPLC System with the prescribed monograph conditions.3 The column dimensions and method conditions were then scaled to a ultra-high-performance liquid chromatography (UHPLC) column with smaller particle size, inside diameter (I.D.), and length using the Waters Column Calculator. Resolution, tailing, and RSDs for peak area and retention time were used to assess the chromatographic performance of the scaled methods. To verify the quantitative performance of the scaled methods, the amount of API and impurity in an unknown sample was determined.
Scaling of the isocratic assay method
The USP isocratic assay method for quetiapine fumarate was scaled to a 3.0×150 mm, 2.5 µm particle column with a maximum column pressure of 18,000 psi. The maximum pressure of the column allows for the flexibility of running at higher flow rates with higher overall system operating pressure on the Alliance iS HPLC System. The Waters Columns Calculator was used to determine the scaled flow rate of 1.10 mL/min and an injection volume of 13 µL (Figure 1).
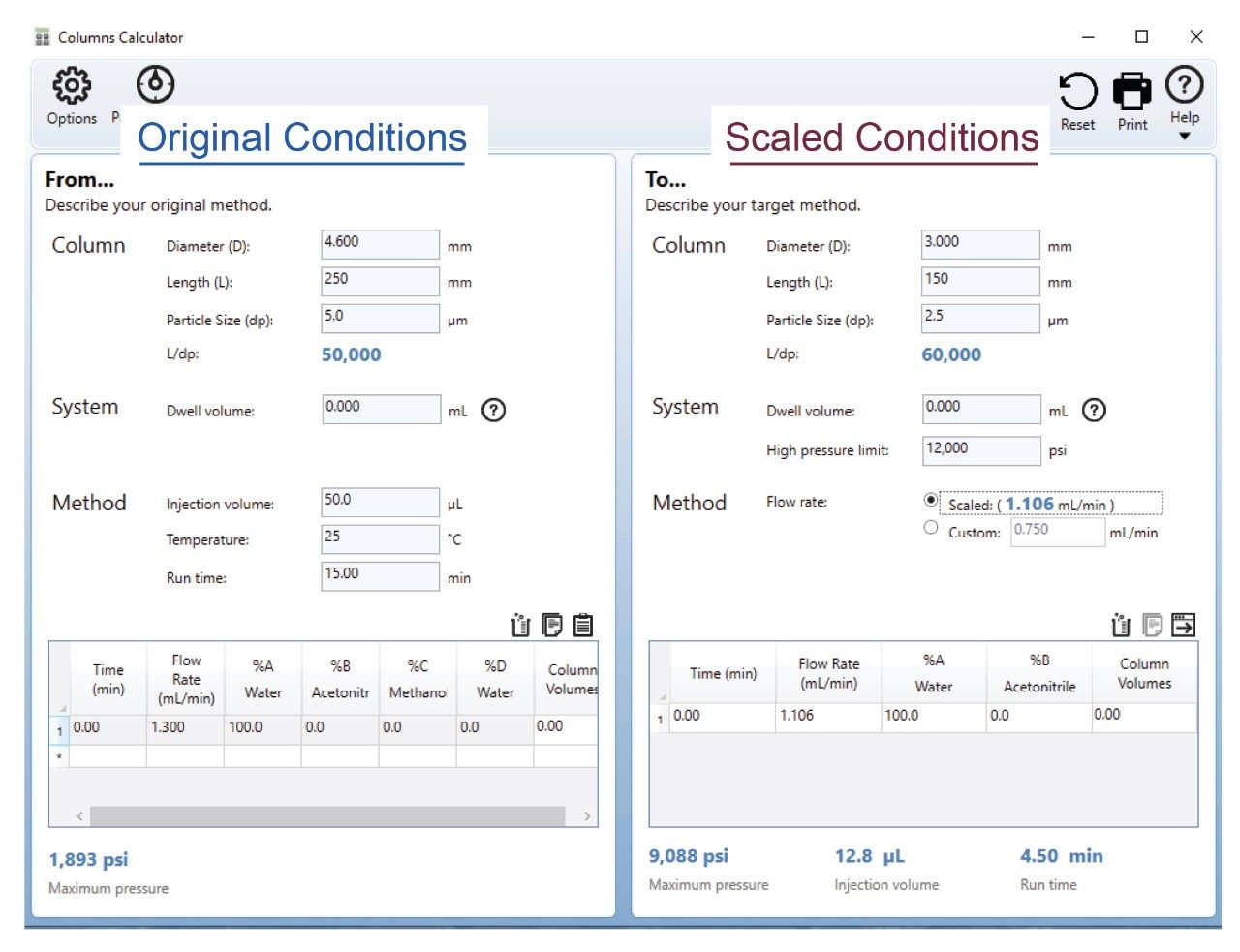
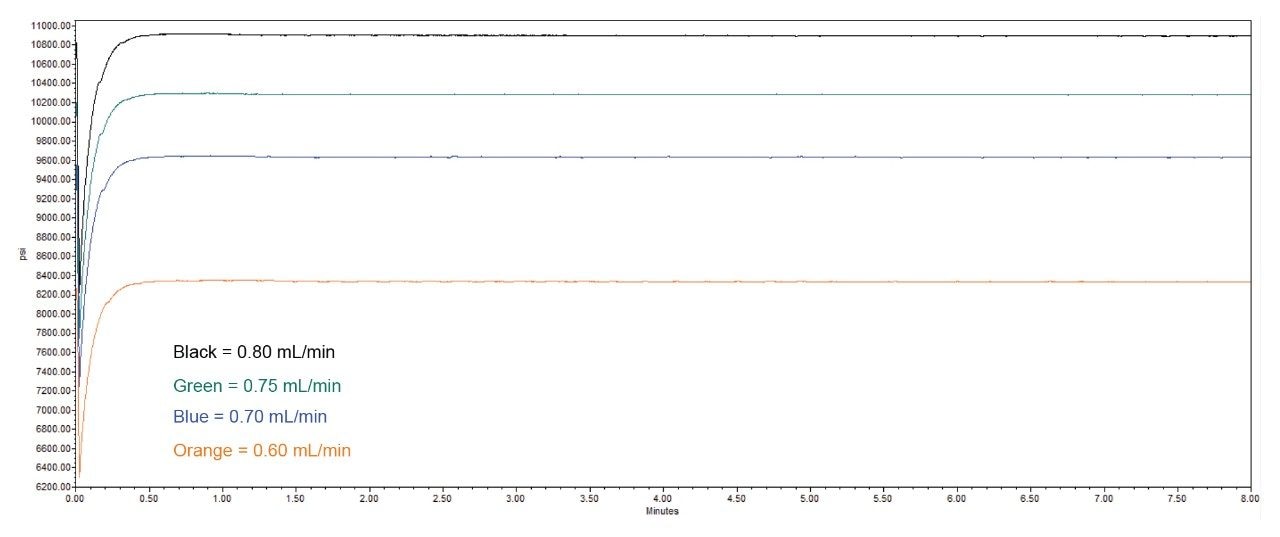
The scaled flow rate of 1.10 mL/min needed to be decreased to a lower flow rate since the Alliance iS HPLC System was unable to reach that flow rate without over-pressurizing the system. In order to determine the flow rate for the scaled method, the flow on the system started at a rate 0.60 mL/min and gradually increased to 0.80 mL/min before reaching maximum pressure of the system (Figure 2). With this information, the assay was run at 0.60 mL/min, 0.70 mL/min, 0.75 mL/min, and 0.80 mL/min to make sure each flow rate was able to meet the system suitability requirements. The flow rate of 0.75 mL/min was decided as the final flow rate for the scaled assay method for the quetiapine assay method since it had a system operating pressure of ~10,500 psi and was the highest flow rate the system was able to reach without being too close the limit of 12,000 psi.
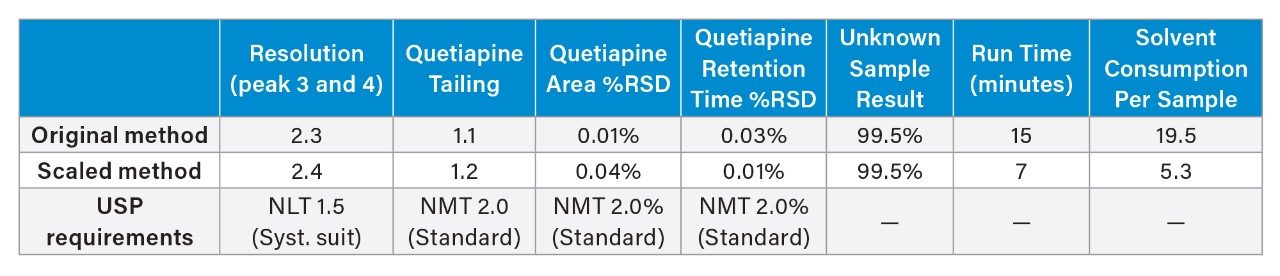
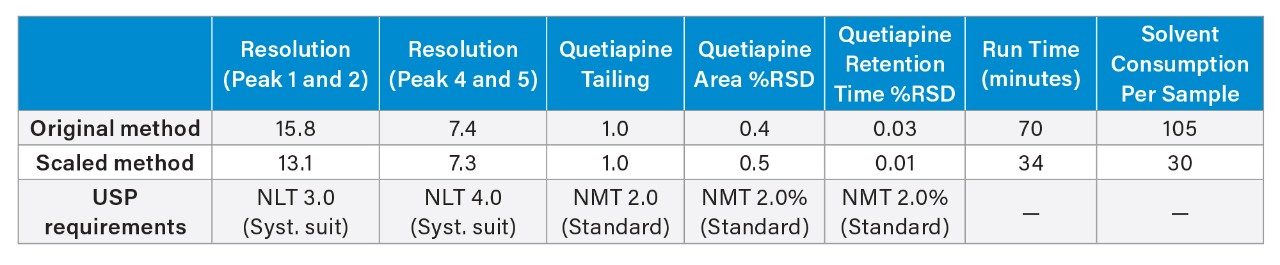
Results for six replicate injections of the standard solution and the system suitability solution are shown in Table 2 for the assay method analyzed with the flow rate of 0.75 mL/min. The resolution, tailing, and RSDs for peak area and retention time for the original HPLC method analysis and the scaled UHPLC method analysis on the Alliance iS HPLC System showed similar chromatographic performance (Table 3). Also, the quantitative percent of the API in the sample of quetiapine that was calculated was similarly produced on both the original and scaled USP method. Scaling the original assay method decreased the run time by 57% and the solvent consumption by 71% while maintaining the separations quality and quantitative results. Chromatograms of the system suitability solution for the assay method are shown in Figure 3.
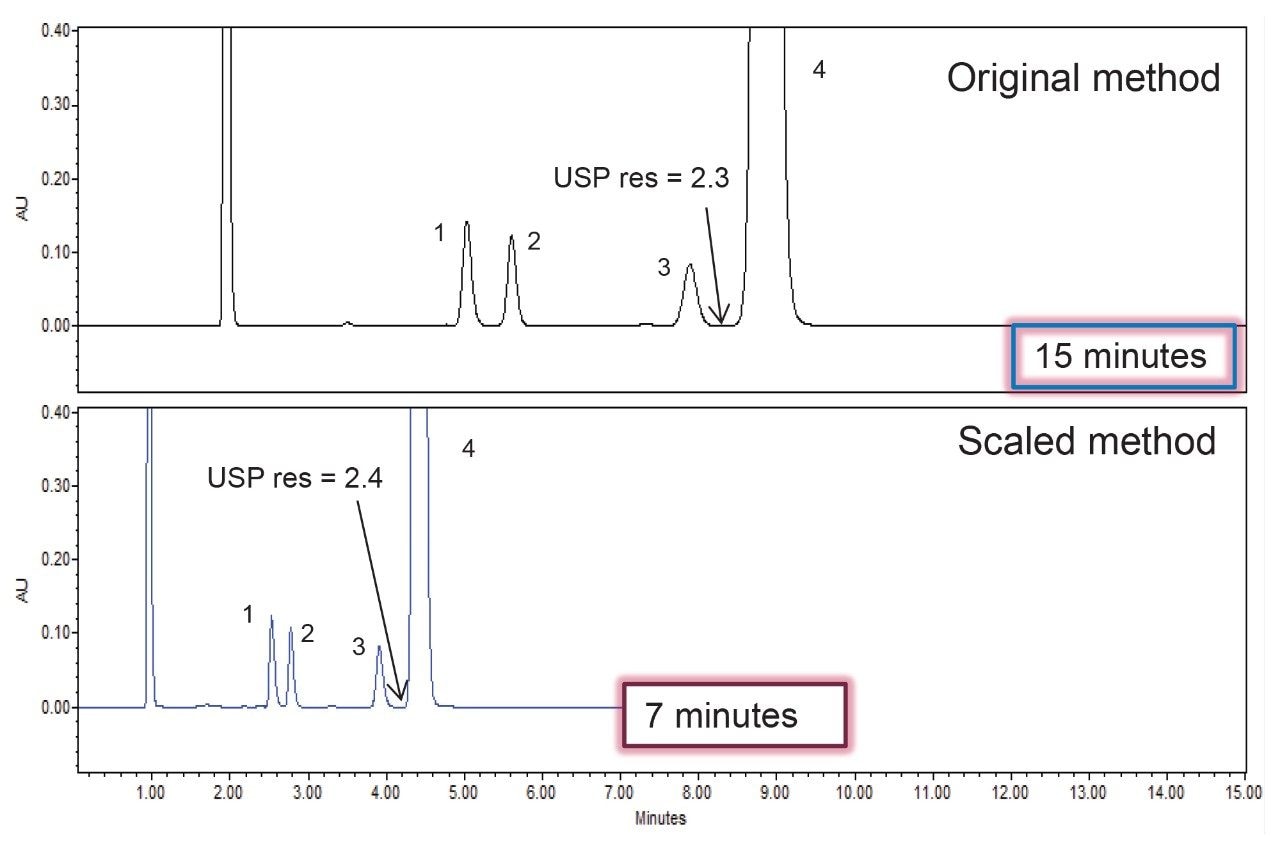
Scaling of the gradient impurities method
The USP quetiapine impurities method was also scaled from the original HPLC column to a 3.0×100 mm, 2.5 µm particle column using the Waters Columns Calculator. The scaled method conditions have a flow rate is 0.90 mL/min with an injection volume of 6 µL. In order to determine the scaled gradient time, the Waters Column Calculator calculates the timing steps according to column volumes.6 The results for 6 replicate injections of the standard solutions are shown in Table 4. The original HPLC method and the scaled UHPLC method showed comparable chromatographic performance in terms of resolution, tailing, and peak area and retention time RSDs. Scaling the original USP impurity method to a 2.5 µm particle column decreased the run time by 51% and the solvent usage by 71%. Chromatograms of the system suitability solution for the impurity method are shown in Figure 4.
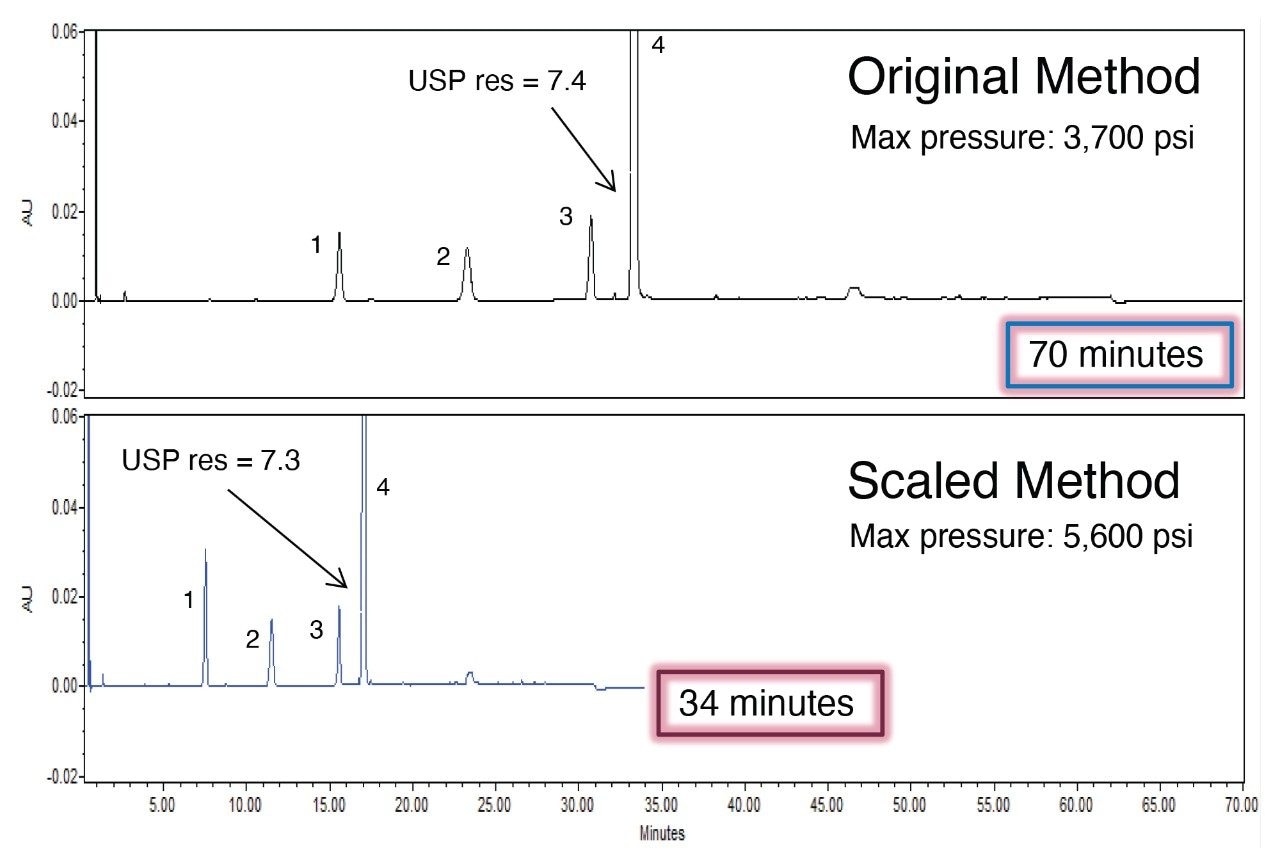
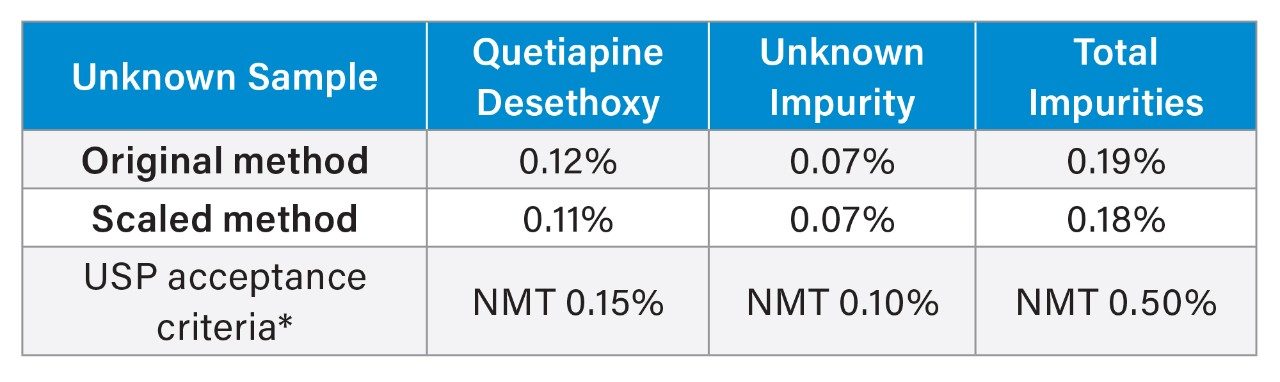
An important part of method scaling is the ability to generate the same results using either the original or the scaled method conditions. The quantitative performance of the scaled impurity method was assessed calculating the percent of two impurities found in the sample according to USP monograph.3 The unknown sample contained two impurity peaks, quetiapine desethoxy and an unknown impurity. The calculated results for the original method and the scaled method are in Table 5. The HPLC method conditions and the scaled UHPLC method conditions were able to produce similar quantitated amounts for the quetiapine desethoxy and the unknown impurity peaks. Scaling the USP quetiapine fumarate impurities method on the Alliance iS HPLC System produced equivalent quantification of impurities contained within a sample of the API. This demonstrates that scaled methods can be used to generate reliable data.
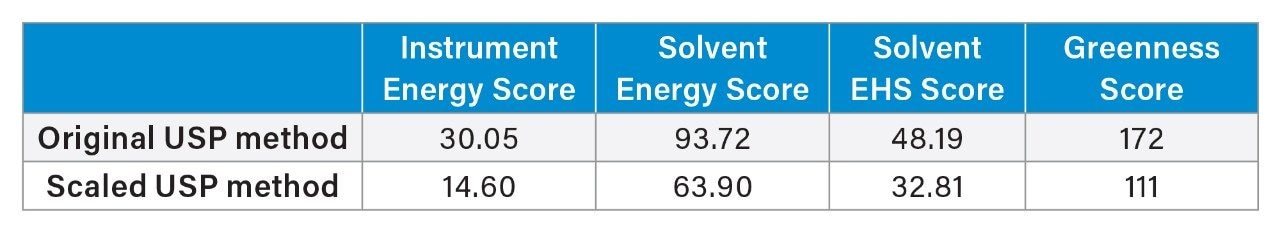
When modernizing USP methods, the AMGS can be used to calculate the “Greenness Score” for the USP quetiapine fumarate impurities method for both the original method and the scaled method. This will provide a measure of sustainability to prove that modernizing older monograph methods not only provides the benefits of lower run times and less solvent used, but also greatly improves the sustainability of running one such assay.5 The results of using the AMGS calculator can be found in Table 6. As expected, the original USP HPLC method has a higher instrument energy score, solvent energy score, and solvent EHS score. These values result in a Greenness Score of 172, while the scaled USP method results in a Greenness Score of 111 which helps to improve the sustainability of the laboratory.
Conclusion
Using the method scaling guidelines within the USP General Chapter <621>,1 traditional isocratic and gradient HPLC methods are capable of being scaled to columns with smaller particle sizes and shorter lengths in order to significantly decrease run time and solvent consumption while still providing the same chromatographic and quantitative performance. Scaling a USP method enables the use of modern column chemistries and modern LC hardware to deliver improved throughput with decreased solvent consumption all while providing accurate and reproducible chromatographic data. Both the isocratic quetiapine fumarate assay method and the gradient quetiapine impurities method were successfully scaled to smaller particle size columns using the Waters Columns Calculator on the Alliance iS HPLC System. By taking advantage of the higher-pressure limits of the system, scaling to 3.0 mm ID, 2.5 µm columns was achievable. The scaled isocratic and gradient methods maintained similar chromatographic performance in terms of resolution, peak tailing, and retention time and peak area RSD. Additionally, quantitative results for the calculated API and the calculated impurities contained in the API sample were consistent for the original and scaled methods. Using the AMGS metric comparing the original USP impurity method to the scaled USP quetiapine impurity method, it was concluded that the use of the scaled USP quetiapine impurity method improves the “Greenness Score” of the method and the sustainability of the laboratory.
References
- Berthelette Kenneth D., Walter Thomas, Collins Chris, DeLoffi Maureen, Haynes Kim. Applying Analytical Method Greenness Scoring to the USP Monograph of Naproxen; Improving the Sustainability of Validated Methods by Modernizing to Newer Technology. Application Note. 720008366. Waters Corporation. May 2024.
- Chapter <621> Chromatography. United States Pharmacopeia and National Formulary Baltimore, MD: United Book Press, Inc.; 2025.
- Official Monographs, Quetiapine Fumarate USP 40 NF35 S1, United States Pharmacopeia and National Formulary (USP 40-NF35 S1) Baltimore, MD: United Book Press, Inc.; 2017. p. 5939.
- ACS Green Chemistry Institute Pharmaceutical Roundtable Website. https://acsgcipr.org// <https://acsgcipr.org/amgs/> Accessed 30-June-2025.
- Columns Calculator Online Help. Waters Columns Calculator, version 2.0.
- Dlugasch Amanda B., Simeone Jennifer, McConville Patricia R. Scaling a USP Gradient Method on the ACQUITY Arc System in Support of Life Cycle Management. Application Note. 720006620. Waters Corporation. July 2019.
Featured Products
720008973, August 2025