Quantification of Ergosterol Using Microwave-Assisted Extraction and a Rapid 5-Minute Isocratic HPLC Method
Abstract
Functional mushroom products have gained popularity in recent years, making their way onto store shelves in the form of supplements, drinks, foods, and other products. The compound ergosterol, which is found in all mushrooms, has been claimed to have several health benefits, including converting into vitamin D2 when exposed to UV light. The determination of ergosterol generally requires saponification and then extraction using lengthy procedures. In this application note, saponification was avoided by using a rapid and easy microwave-assisted extraction. The quantification of ergosterol utilized an Arc™ Premier System with a fast, 5-minute isocratic elution on an XBridge™ Premier BEH™ C18 Column with an ACQUITY™ 2998 Photodiode Array (PDA) Detector. A sample containing a mixture of functional mushrooms was analyzed using the method developed here. Empower™ Software’s Peak Purity tool confirmed that no peaks coeluted with ergosterol.
Benefits
- The CEM Discover Prep System allowed for a rapid, easy sample preparation that avoided saponification
- A rapid, 5-minute isocratic method reduced solvent consumption, leading to an overall greener method
- The Empower Software Peak Purity tool was utilized to confirm all peaks were well-separated in the final method
Introduction
Functional mushrooms are a popular nutraceutical, with a variety of benefits being claimed for different fungal species. The numerous compounds contained in different species, coupled with the lack of guidelines in the supplement market, have contributed to the challenge in the development of quality analytical methods. Ergosterol is a compound that is common to all fungi and health benefits such as reduction in pain related inflammation, reduced incidences of cardiovascular disease, antioxidant, antimicrobial, and anti-tumor activity have been reported.1-5 Additionally, ergosterol can act as a quality indicator, and can be used as a vitamin D2 supplement if it is exposed to UV light.6 Usage as a vitamin D2 supplement has been approved for certain foods, such as cereals, by the US FDA.7
Currently, there is a wide range of methods for the extraction and quantification of ergosterol in mushrooms. A variety of sample preparation methods have successfully extracted ergosterol, some of which include Soxhlet extractions, ultrasound-assisted, microwave-assisted, and supercritical fluid extractions. Most of these methods require a saponification step before the extraction, but some, such as microwave-assisted extractions, can achieve good results without using this long and tedious procedure.8
The work described here produced a rapid isocratic method able to separate and quantitate ergosterol from a variety of functional mushrooms. The ergosterol was extracted from mushroom samples using an optimized microwave-assisted extraction method. The ACQUITY 2998 PDA Detector was used to identify and quantify ergosterol in the mushroom samples. Spectral homogeneity of ergosterol was evaluated using the 3D PDA data coupled with the Peak Purity tool in Empower Software. A linear range was established and used to determine the concentration of ergosterol in the functional mushroom extracts.
Experimental
Sample Preparation
Extractions were carried out following an optimized microwave digestion CEM Discover Prep System.9 Mushroom powder was obtained from a local GNC health and supplement store. The dry mushroom powder contained a mixture of lion’s mane, turkey tail, reishi, cordyceps, maitake, and chaga. A 40-mg sample was weighed out and added to 25 ml of HPLC-grade, denatured ethanol in the discovery 2.0’s reaction vessel with a small stir bar. A microwave method was programmed to the parameters seen in the microwave digestion conditions table. While the maximum pressure on the method was set at 300 psi, the pressure was approximately 100 psi for most of the extraction. The extract was filtered through a 0.2-µm nylon filter. The solution was then dried under a flow of nitrogen. The dry amber oil extract was then solvated at 2 mg/ml in HPLC-grade, denatured ethanol with 10% dimethyl sulfoxide (DMSO).
Sample Description
Ergosterol was obtained from Sigma Aldrich (Darmstadt, Germany). Stock solution of ergosterol was prepared in methanol with 10% DMSO diluted for standards to a concentration ranging from 0.001 to 0.1 mg/ml.
Microwave Digestion Conditions
|
Instrument: |
CEM Discover Prep System, CEM Corporation, NC, USA |
|
Temperature: |
132 °C |
|
Time: |
20 min |
|
Pressure: |
300 psi |
|
Power: |
300 W |
|
Stirring: |
Medium |
LC Conditions
|
LC system: |
Arc Premier QSM-r |
|
Detection: |
ACQUITY PDA 2998 @ 280 nm |
|
Vials: |
1mL Total Recovery Vial, p/n: 186000385DV |
|
Column(s): |
XBridge Premier BEH C18 2.5μm 4.6 x 50 mm p/n: 186009847 |
|
Column temperature: |
60 °C |
|
Injection volume: |
10 µl |
|
Flow rate: |
1 ml/min |
|
Mobile phase: |
0.1 % formic acid in 12:88 Water:Acetonitrile |
Data Management
|
Chromatography software: |
Empower 3.8.0 |
Results and Discussion
An Arc Premier System with a gradient method was used for screening of both an XBridge Premier BEH C18 2.5µm x 4.6 x 50 mm and an XSelect Premier CSH™ C18 2.5µm x 4.6 x 50 mm Column. The XBridge BEH C18 Column provided a better separation and was used for further method development. Column screening was conducted with a 5-99%B gradient over 20 minutes. Initial screening conditions were able to produce a good separation that outperformed the resolution and tailing requirements of the desired method. Further method optimization consisted of improving the run time, peak tailing, and peak height.
Due to only having one analyte with no close-eluting compounds, isocratic elution methods were tested. Based on the screening runs, three isocratic methods were screened at 88%B, 89%B, and 90%B. The 88%B isocratic elution retained ergosterol long enough to be outside any baseline disturbances from the column front. The final elution can be seen in Figure 1. This method provided a resolution greater than 2.5 with a peak tailing factor of 1.0.
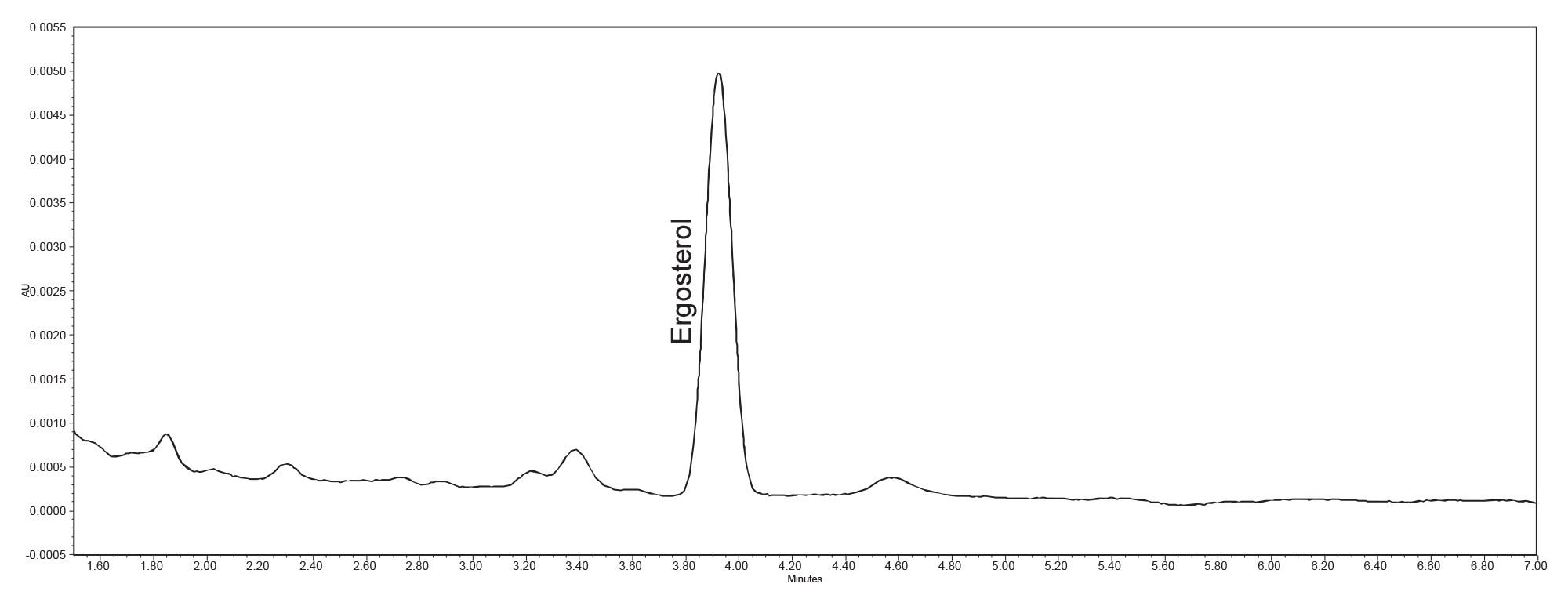
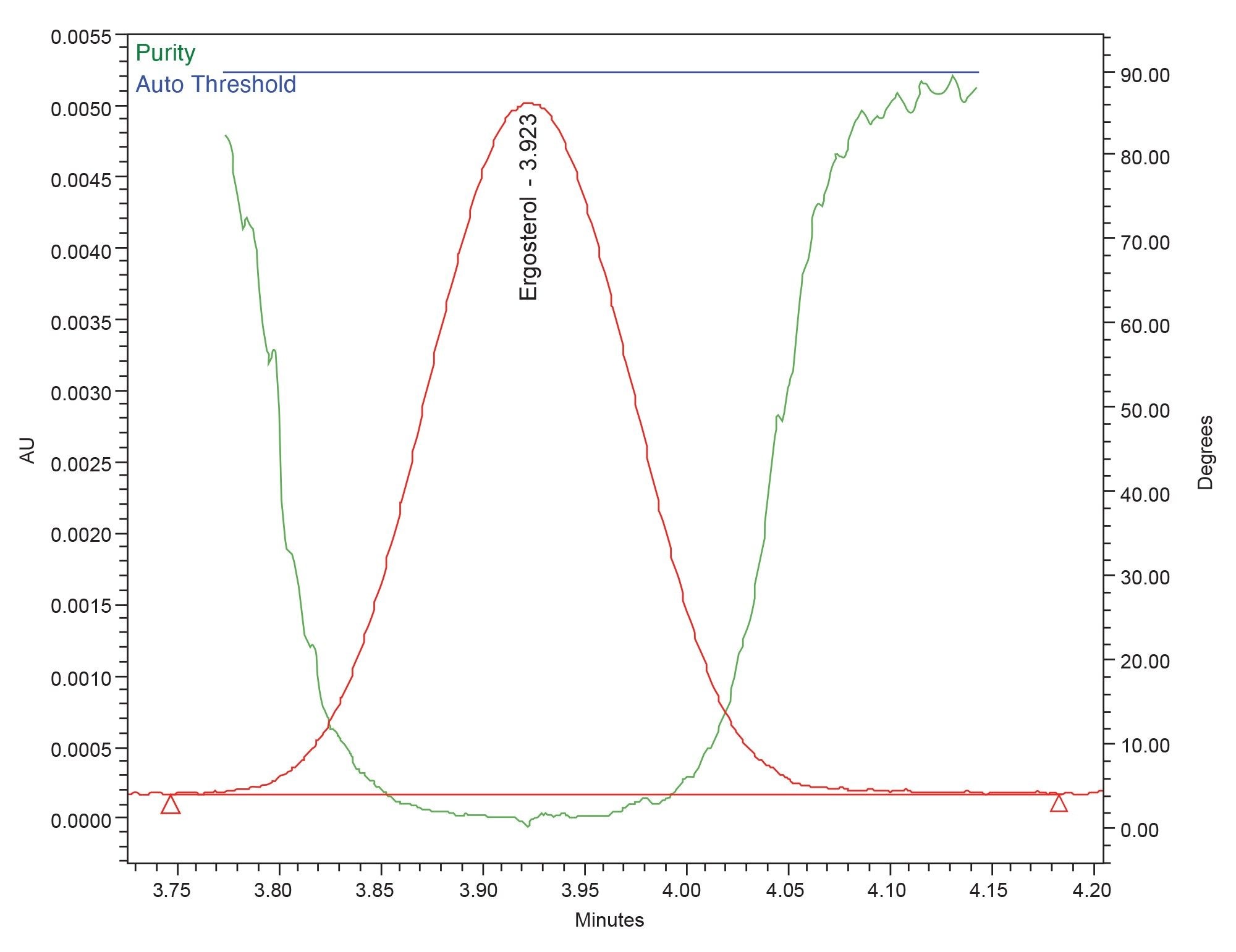
Ensuring chromatographic peaks of interest do not contain any coeluting analytes is an important aspect to consider during method development. Generally, peak purity determination is conducted using either mass spectrometry or PDA detection. Since sterol compounds such as ergosterol are not easily ionized under electrospray conditions, the PDA was used to determine peak purity. The results of the peak purity analysis in Figure 2 show a peak purity angle less than the automatic threshold calculated by Empower Software, thus proving that the ergosterol peak is spectrally homogeneous, which suggests there are no analytes coeluting with the ergosterol peak.
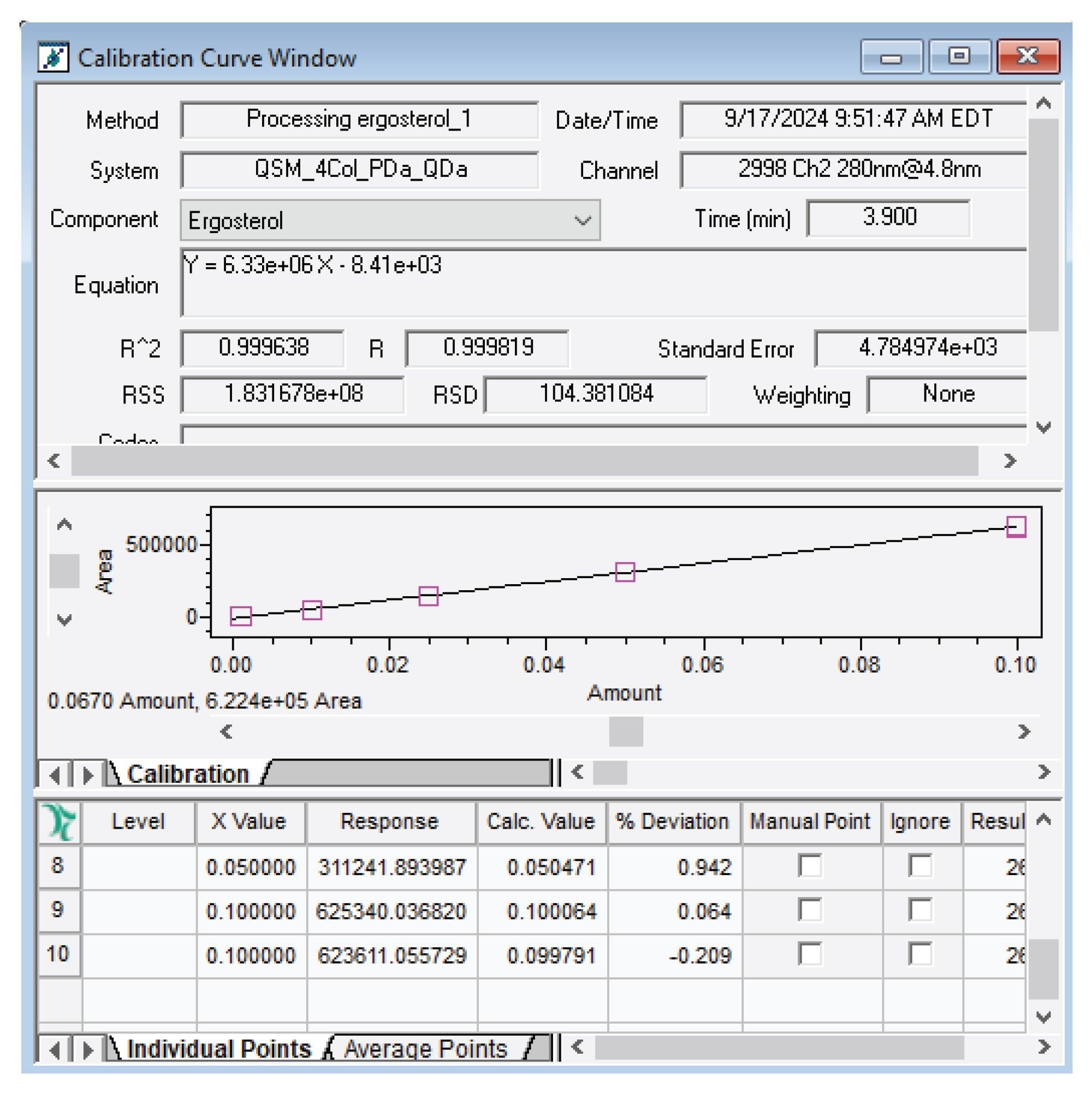
To determine the concentration of ergosterol in the mushroom extract, a calibration curve was created. The method provided a linear range from 0.001-0.1 mg/ml, this encompasses the expected range of ergosterol in previously published data, which ranges from ~298-1245 mg/100 g dry mushroom (calculated at ~0.006-0.024 mg/ml using this sample preparation method).10 The extract in this study was found to contain about 305 mg of ergosterol per 100 g of dry mushroom powder. The linear curve used for this calculation can be seen in Figure 3.
Conclusion
A fast method for the separation and determination of ergosterol content in mushrooms was developed utilizing an Arc Premier UHPLC System with PDA detection. A CEM Discover Prep System was used for rapid extraction while avoiding the lengthy saponification step. Empower Software’s Peak Purity tool, in combination with the 3D PDA spectrum collected, were able ensure the ergosterol to be spectrally pure. The linearity of the ergosterol allows for quantification within the expected ranges produced by mushrooms with this extraction.
References
- S. Shao, M. Hernandez, j. Kramer, D. Rinker, R. Tsoa. Ergosterol Profiles, Fatty Acid Composition, and Antioxidant Activities of Button Mushrooms as Affected by Tissue Part and Developmental Stage. J. Agric. Food Chem. 2010, 58: 11616–11625.
- K. Yasukawa, T. Aoki, M. Takido, T. Ikekawa, H. Saito, T. Matsukawa. Inhibitory Effects of Ergosterol Isolated from the Edible Mushroom Hypsizius marmoreus on TPA-induced Inflammatory Ear Oedema and Tumor Promotion in Mice. Phytother. Res. 1994, 62: 10–13.
- J. Yang, L. Mills, G. Nair. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50: 3479–3482.
- L. Fan, H. Pan, T. Soccol, A. Pandey, R. Soccol. Advances in mushroom research in the last decade. Food Technol. Biotech. 2006, 44: 303–311.
- T. Ravi Subbiah, W. Abplanalp, Ergosterol (Major Sterol of Baker’s and Brewer’s Yeast Extract) Inhibits the Growth of Human Breast Cancer Cells in Vitro and the Potential Role of its Oxidation Products. Int. J. Vitam Nutr. Res. 2003, 73: 19–23.
- J. Barreira, M. Oliveira, I. Ferreira. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Ana. Met. 2014, 7(1): DOI:10.1007/s12161-013–9621-9.
- FDA. Food Additives Permitted for Direct Addition to Food for Human Consumption (Vitamin D2 Mushroom Powder) 21 C.F.R S172.382. U.S. Food and Drug Administration. 2020.
- K. Papoutsis, S. Grasso, A. Menon, N. Brunton, J. Lyng, J. Jacquier, D. Bhuyan. Recovery of Ergosterol and Vitamin D2 from Mushroom Waste – Potential Valorization by Food and Pharmaceutical Industries. Trend in Food Science & Technology. 2020, 99: 351–366.
- S. Heleno, M.A. Prieto, L. Barros, A. Rodrigues, M. Barreiro, I. Ferreira. Optimization of Microwave Assisted Extraction of Ergosterol from Agaricus bisporus L. By-products Using Response Surface Methodology. Food and Bioproducts Processing. 2016, 100: 25–35.
- K. Phillips, D. Ruggio, R. Horst, B. Minor, R. Simon, M. Feeney, W. Byrdwell, D. Haytowitz. Vitamin D and Sterol Composition of 10 Types of Mushrooms from Retail Suppliers in the United States. J. Agric. Food Chem. 2001,59: 7841–7853.
720008652, December 2024