To develop a method on the ACQUITY UPLC H-Class system using an ACQUITY UPLC BEH Amide sub-2 μm particle stationary phase in HILIC mode to successfully resolve and analyze metformin and six related substances.
Metformin hydrochloride is an anti-diabetic drug typically administered orally while treating non-insulin dependant (type 2) diabetes mellitus.1 It is one of the most widely-used oral antidiabetic drugs worldwide, with over 48 million generic formulation prescriptions filled in the United States alone.2 Liquid chromatographic analysis of metformin and related impurities presents a challenging task due to the highly polar characteristics of the molecules and the low UV absorbance of the analytes. These challenges limit the options to manipulate selectivity during method development, especially with reversedphase (RP) chromatography. Numerous methods are cited utilizing a variety of techniques such as ion chromatography, hydrophilic interaction chromatography (HILIC), and RP LC methodologies reporting run times up to 30 minutes.1,3,4
In this application note, a method was developed on the ACQUITY UPLC H-Class system using an ACQUITY UPLC BEH Amide sub-2 μm particle stationary phase in HILIC mode to successfully resolve and analyze metformin and six related substances. Development of the method was facilitated through the column and solvent switching capabilities of the ACQUITY UPLC H-Class which allows automated changes of stationary phase, ionic strength, cation buffer, pH and temperature. The major contributors to the successful separation of metformin and the related substances are discussed. A routine use evaluation study was performed to determine feasibility of the method for use in QC laboratories. Informatics provided visualization of trending results with intent to identify deficiencies regarding the developed methodology. The final method will provide cost reduction improvements in method robustness for routine analysis.
Sample Description
Samples were provided by a pharmaceutical collaborator. Stock solutions of metformin hydrochloride, as well as impurities A, B, C, D, E and I were prepared in water. Working standards were prepared as per the previous HPLC methodology (70:30 acetonitrile:water). A working standard mixture was prepared whereas impurity concentrations were in respect to the metformin concentration: Impurity A was prepared at 0.05% of metformin and Impurities B, C, D, E and I were prepared at 0.1% of metformin. A mixture of the impurities without addition of metformin was also prepared at the same concentration as the impurities working standard. In addition to the working standard and impurity working standard, two separate preparations consisting of vials prepared with metformin with and without impurities spiked into the matrix. Individual standards were also prepared for each of the analyte constituents.
|
Instrument: |
ACQUITY UPLC H-Class configured with CM-A, CM-AUX, SSV, PDA |
|
Buffer: |
20 mM potassium phosphate, pH 2.3 |
|
Mobile Phase: |
80:20 acetonitrile:buffer |
|
Separation Mode: |
Isocratic |
|
Detection: |
UV at 218 nm |
|
Column: |
ACQUITY UPLC BEH Amide, 2.1 x 150 mm, 1.7 μm, part number 186004802 |
|
Needle Wash: |
90:10 acetonitrile:water |
|
Seal Wash: |
90:10 water:methanol |
|
Sample Diluent: |
70:30 acetonitrile:water |
|
Flow Rate: |
0.5 mL/min |
|
Column Temp.: |
40 °C |
|
Injection Volume: |
1.0 μL |
|
Data Management: |
Empower 2 CDS |
The originally supplied HPLC methodology for metformin utilized isocratic conditions with a low-pH sodium phosphate buffer and acetonitrile mobile phase.1 An Atlantis HILIC 4.6 mm x 250 mm, 5 μm column was used with an approximate flow of 2 mL/min resulting in a run time of 30 minutes. Sample injection volume was 10 μL. The HPLC method resolves metformin and all six impurities (not shown).
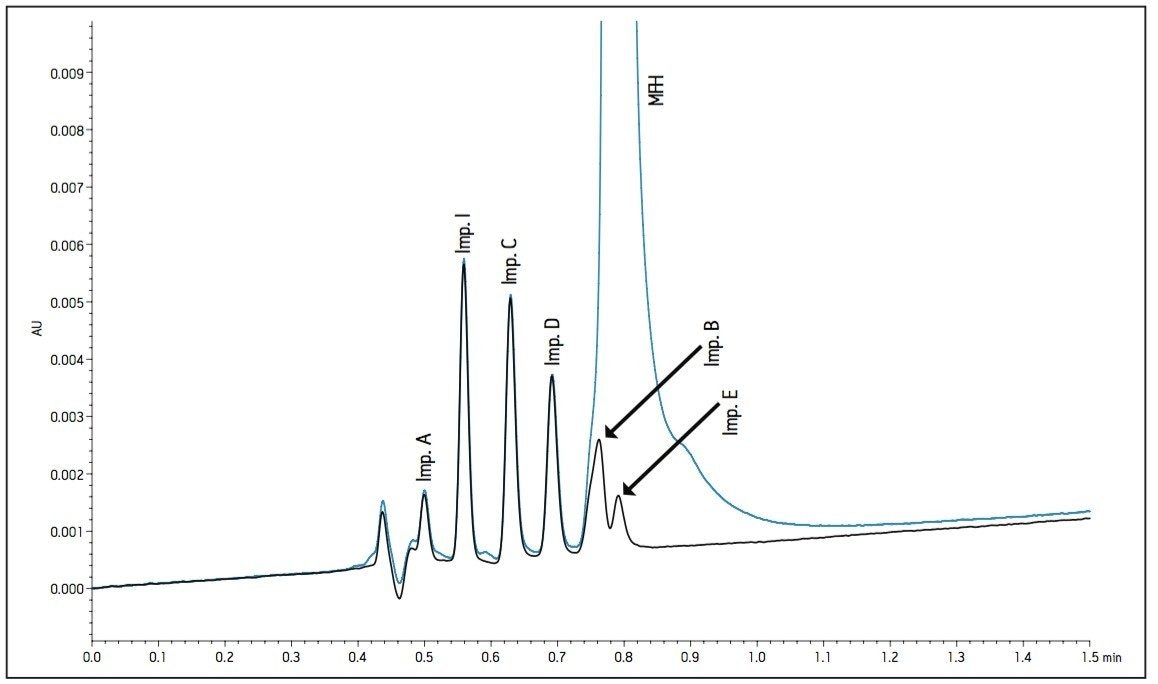
The HPLC methodology was transferred to a 2.1 x 100 mm, 1.7 μm ACQUITY UPLC BEH HILIC column using the ACQUITY UPLC Columns Calculator. It should be noted that the ACQUITY UPLC BEH HILIC stationary phase does not have exactly the same selectivity as the Atlantis HILIC stationary phase due to differences in the base particle, although the Waters Column Selection application indicated that the two stationary phases have similar selectivity. In this application, there were observed selectivity differences between the Atlantis HILIC column and BEH HILIC column. The chromatogram in Figure 1 shows coelutions and a lack of overall retentivity on the BEH HILIC column using UPLC technology. Slight changes in organic composition were not successful in resolving the impurity peaks. In some instances, as organic composition was increased, salt in the mobile phase precipitated due to mixing a high buffer concentration with a high composition of organic mobile phase. The precipitated salt resulted in increased pressure and baseline absorbance issues. It was then determined that a small amount of redevelopment would be needed to resolve the metformin and related substances by exploring suitable variables.
A method development scheme to analyze metformin and related substances presents a challenging task. Limitations regarding the low UV spectral absorbance of the analytes at 218 nm inhibit the use of typical MS-friendly buffers such as ammonium formate and ammonium acetate, since their UV cutoff approaches 230 nm. Reversed-phase LC is unsuccessful in retaining the analytes due to the polar basic characteristics of the compounds.
A method development scheme was employed to investigate two HILIC stationary phases: ACQUITY UPLC BEH HILIC and ACQUITY UPLC BEH Amide. Ionic strength, buffer cation selection, and temperature were determined as the remaining options to alter selectivity. Based on the poor retentivity and resolution of these compounds using the ACQUITY UPLC BEH HILIC stationary phase, the ACQUITY UPLC BEH Amide column was investigated.
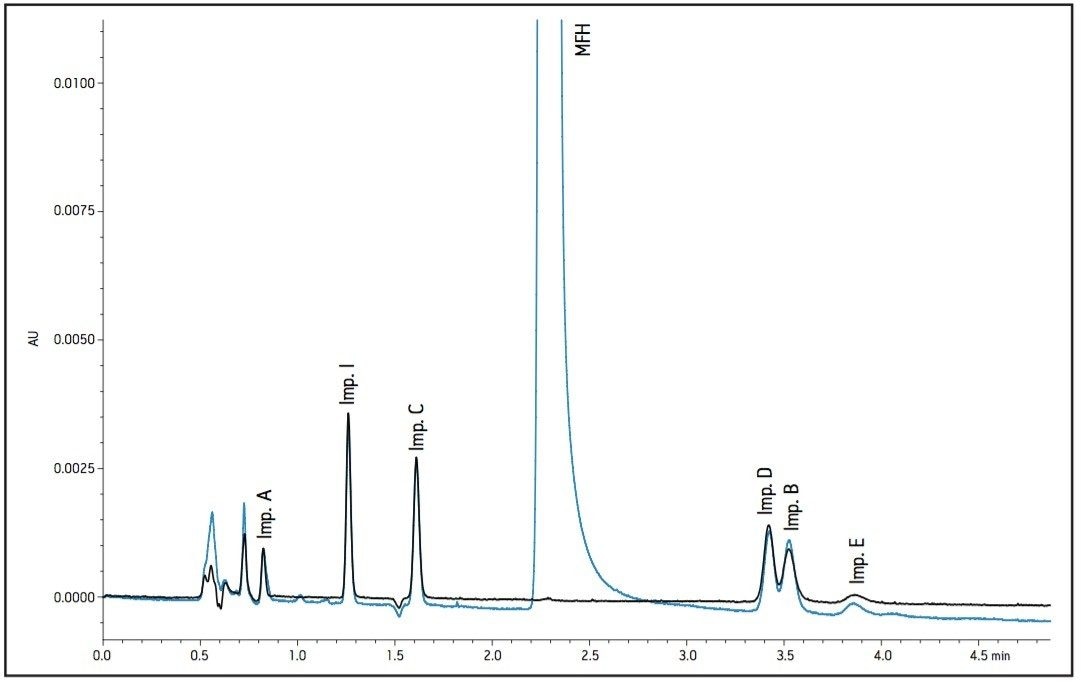
The working standard was injected onto the ACQUITY UPLC BEH Amide 2.1 x 100 mm, 1.7 μm column. The resulting chromatogram in Figure 2 resolved all compounds with the exception of a slight co-elution between Impurity B and Impurity D. Desired improvements in peak shape and sensitivity were seen for Impurity E. Due to the isocratic conditions, changes in flow rate and temperature were explored individually to improve on these critical impurity peaks of interest. An experiment utilizing higher mobilephase pH was explored but the results yielded little to no retention of many of the impurity peaks. The following relationships were observed during development of the separation on the amide column:
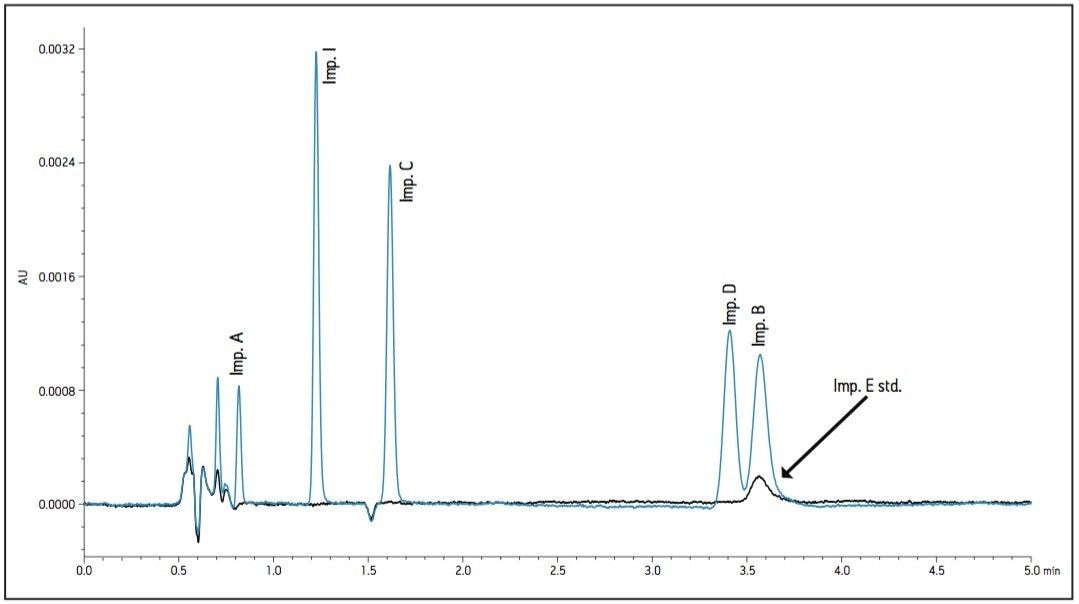
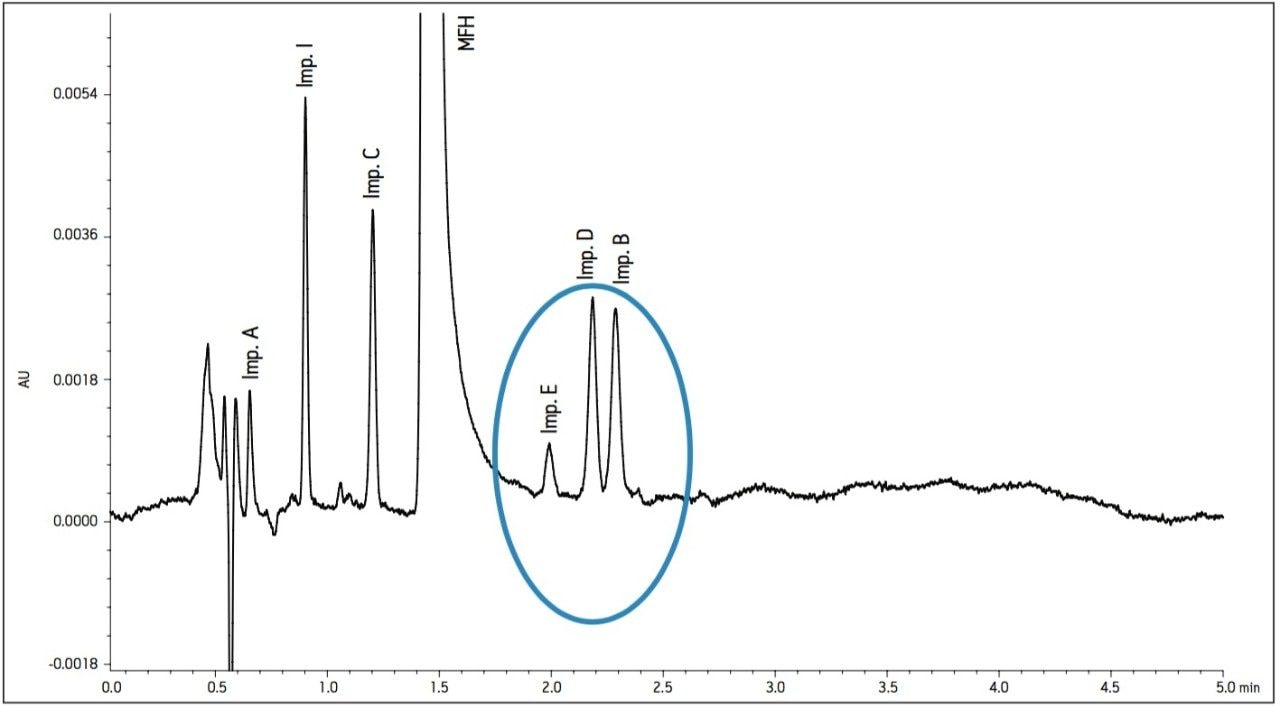
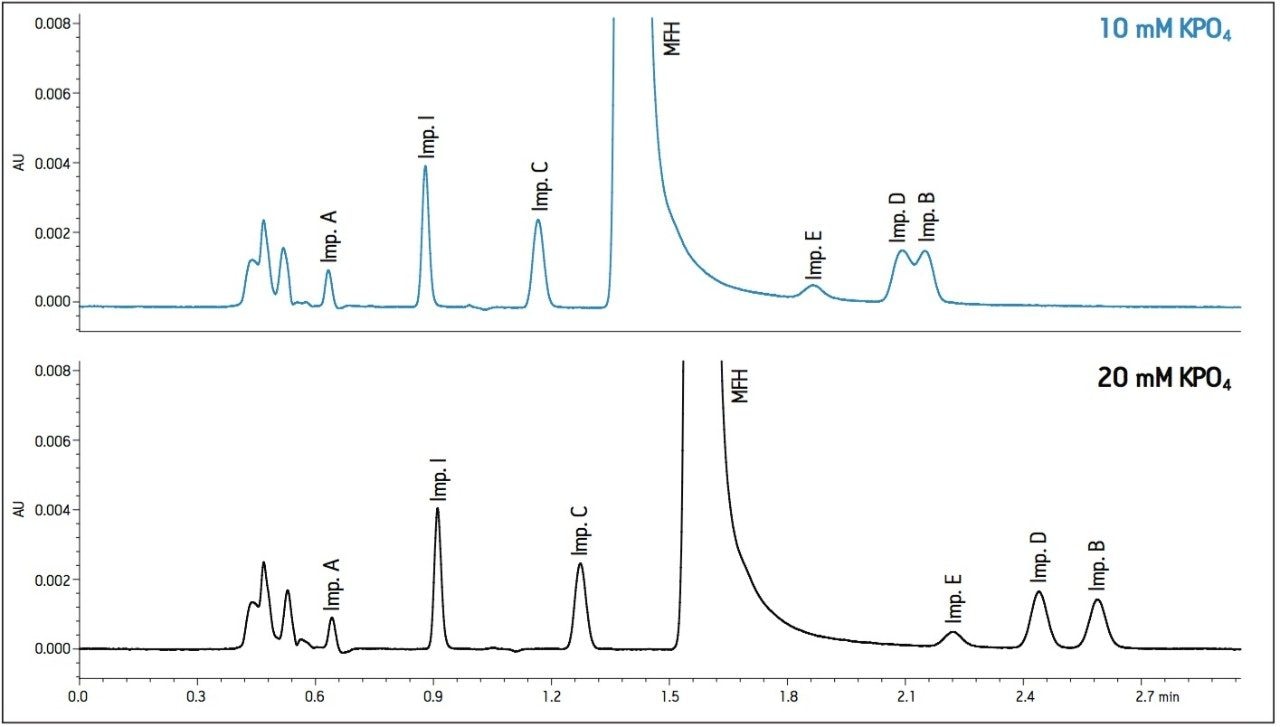
The proper selection of cation in the buffer can help control the ionic interactions on the surface of the column and in some instances, alter selectivity. The 30 mM sodium phosphate buffer was substituted with a 30 mM potassium phosphate buffer while maintaining a pH of 2.2. Optimal conditions were determined by combining the immediate improvement of changing the buffer cation to potassium. Combining the change in cation with a slight adjustment in flow rate and temperature, a desired resolution of the critical peaks was achieved. The chromatogram in Figure 4 shows better peak shape and resolution for Impurity peaks B, D, and E. Also, Impurity E shifted retention and elutes before Impurities B and D. Since the baseline noise was higher with the method in Figure 4, the buffer strength was decreased to minimize the potential for salt precipitation. The effect of decreasing the ionic strength to 10 mM resulted in co-elution of Impurities B and D. A concentration of 20 mM potassium phosphate resulted in acceptable peak shape and resolution (Figure 5).
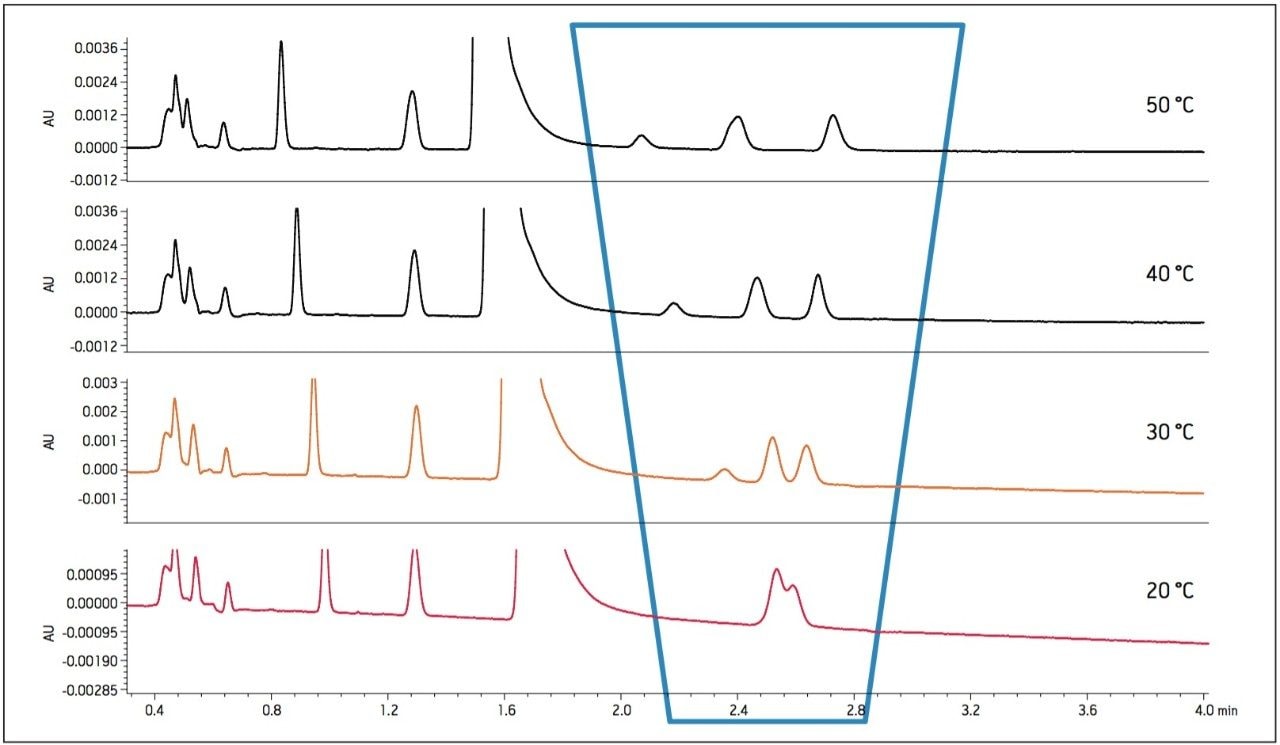
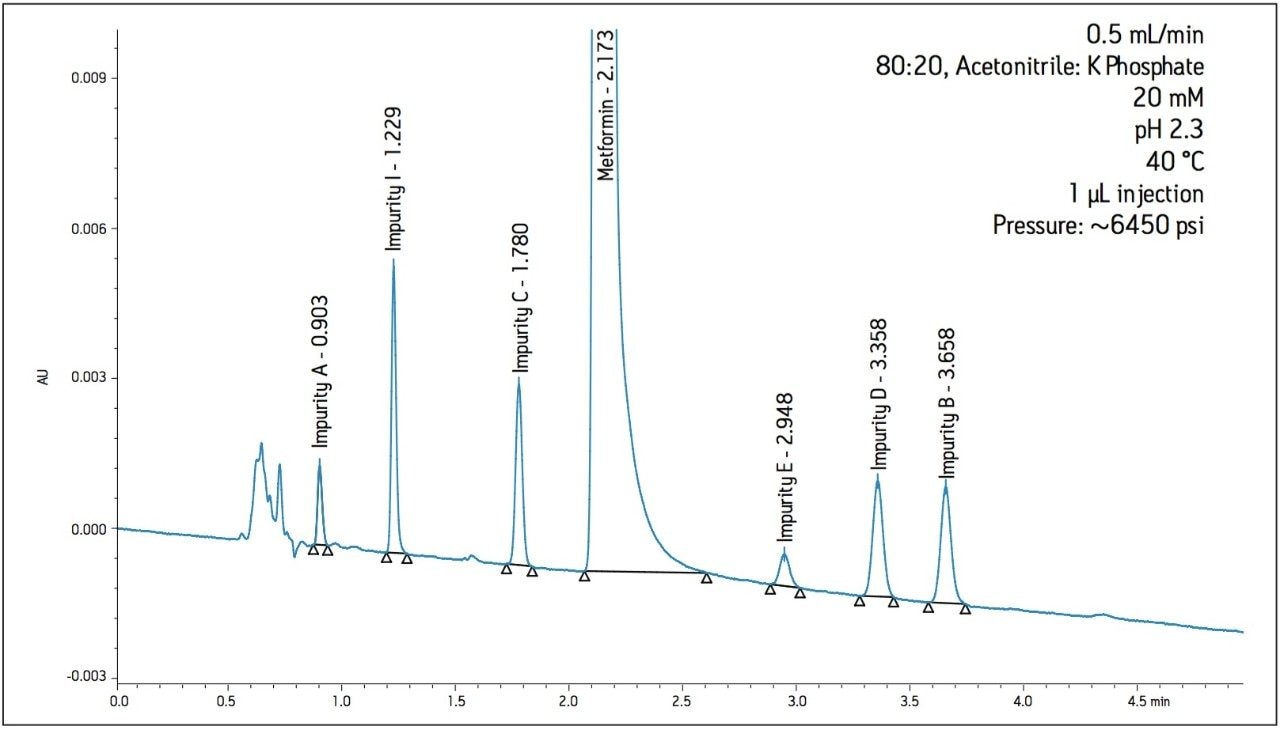
The effects of temperature were explored to determine the effect on selectivity when using the potassium phosphate buffer. Temperature was increased from 20 °C to 50 °C in 10 °C increments. Resolution increased for Impurities B, D, and E as temperature increased. A temperature of 40 °C was determined to provide adequate resolution of the critical pairs (Figure 6). The use of higher temperature resulted in lower column pressure, which allowed the use of a longer column to improve the resolution for the final methodology (Figure 7).
In order to evaluate the effects of using the potassium phosphate buffer at high organic composition, injections of the standards and samples were performed over a period of time to replicate routine use of the method in a QC laboratory. The sample set consisted of a bracketing procedure constructed with the working standard preparations, individual standard preparations, as well as the spiked and unspiked sample matrix formulations totaling over 360 injections for a given experimental run. A single bracket consisted of 30 injections, which was repeated 60 times to achieve 1800 injections to complete the study designed to replicate practices within a quality control testing laboratory. A pre-column filter was installed for preventative and investigative purposes in the event of a pressure increase over the time of the study.
In an effort to understand how the data was trending, custom calculations and custom reports were created in Empower 2 CDS, whereby processed data could be visualized in the form of trend plots without exporting to spreadsheets. Initial pressure readings were approximately 6500 psi and increased steadily to approximately 6700 psi over the first 860 injections, as indicated by the summary pressure trend plot in Figure 8a. Closer investigations of the summary trend data showed further trending within the bracketed sample set (Figure 8b). The trend plots generated in Empower 2 CDS showed increases in system pressure once the matrix samples were injected. This indicated a deficiency in the original sample preparation procedure. The sample preparation procedure was altered to include a longer centrifugation time and the use of 0.2 μm filter disks in place of 0.4 μm filter disks. The filter disks were used to filter the supernatant as it was added to the sample vial. As a result, pressure increases due to the sample preparation was eliminated and extended to a point where the method was suitable for validation.
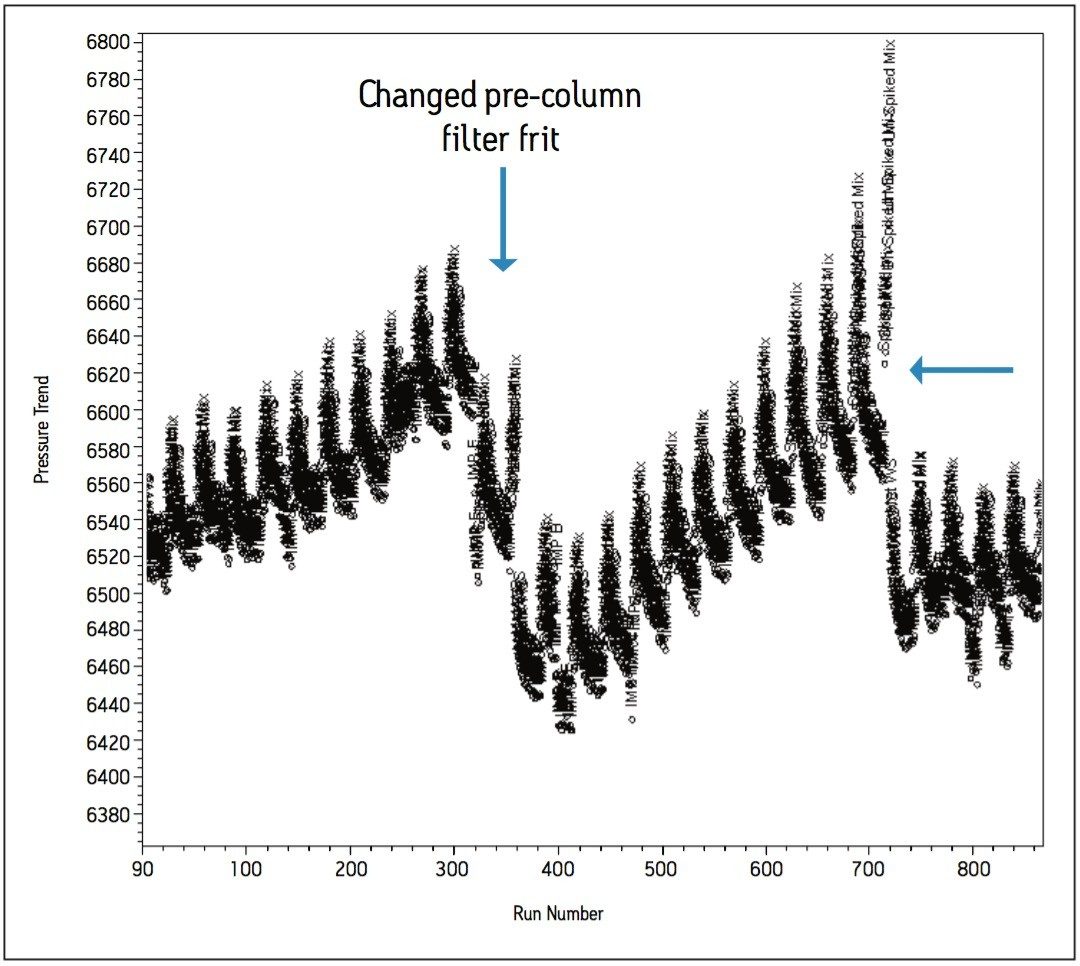
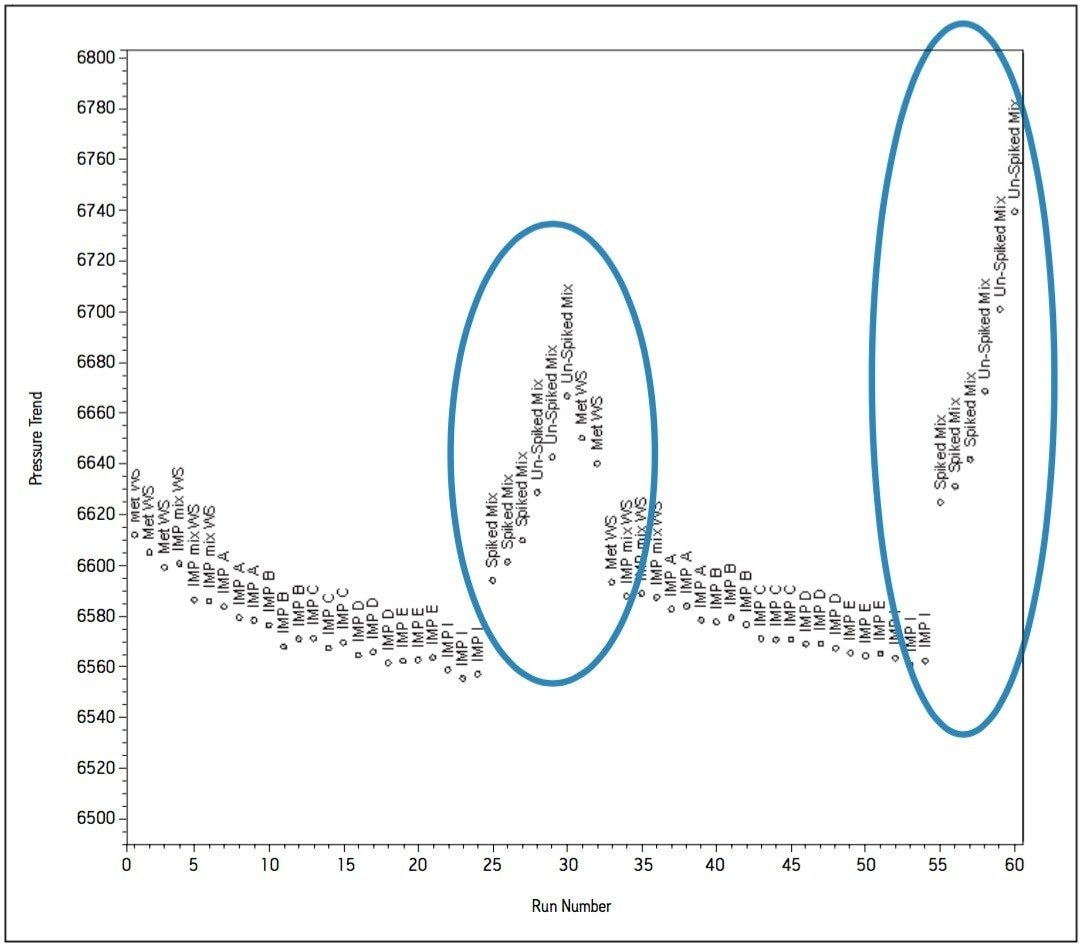
The complete solution consisting of informatics tools, flexible instrumentation, and a selection of chemistries resulted in a method providing a six-fold reduction in analysis time compared to the HPLC methodology. Altering the buffer cation provided a selectivity change between Impurity E and the Impurity pair; B and D. Ionic strength of the buffer influenced the retentivity of Impurities B and D. Temperature was a useful selectivity influence for HILIC method development. The informatics solutions within Empower provided trending insight to effectively troubleshoot issues relating to poor sample preparation. The use of pre-column filters also contributed towards achieving excellent column performance of over 1500 injections.
In retrospect, a Routine Use Study of the original HPLC methodology would be costly. Comparing the mobilephase consumption during 1500 injections on HPLC versus UPLC; HPLC would utilize approximately 65 liters compared to 11 liters consumed using UPLC. At an average cost of $165 per liter acetonitrile, the resulting methodology would save approximately $8800 in solvent consumption. Implementing UPLC technology results in a time savings of 26 days per 1500 injections, or roughly an 80% reduction in analysis time in which the resources can be better utilized to increase profitability.
720004080, March 2013