This work illustrates how the UNIFI Scientific Information System, used with the Xevo TQ-S MS for post-column infusion (PCI), facilitates the determination of matrix factor in bioanalysis studies.
UNIFI Software automates the calculation of both analyte and normalized internal standard matrix factor.
The reliability of analytical data upon which critical toxicological and efficacy findings are based is an essential part of bioanalysis. LC-MS/MS is the technique of choice in quantitative bioanalysis due to its high selectivity and sensitivity, as well as the time savings afforded by significantly faster chromatographic separations and minimized sample preparation.
LC-MS/MS quantitative analysis is influenced by a phenomenon called ion suppression or matrix effects, wherein matrix components present in the biological sample influence the response of the analyte under investigation. The need to adequately address matrix effects data during the method development and validation process has been clearly identified.1-3 This information is reported as matrix factor (MF), which is defined as analyte response in the presence of matrix components divided by analyte response in pure solution.
As drug compounds under investigation become increasingly potent, they require lower doses for efficacy and toxicology assessment. This translates to lower limits of quantitation (LLOQ) during bioanalysis, wherein matrix components in the sample can be present at levels that are much higher than the target analyte. In addition, over the course of pre-clinical and clinical trials, very often a number of samples to be analyzed will contain varying degrees of hemolysis arising from erroneous processing of the blood to plasma. Therefore, it is suggested that hemolyzed samples also be considered during method development and validation to assess any potential effects arising from the matrix. For example, the current EMEA guidelines require that in addition to six unique lots of plasma, hemolyzed plasma should also be tested for matrix effects.
The Waters UNIFI Scientific Information System enables the user to easily quantify matrix factor either by post-column infusion or in a spiked experiment. The software is designed to do all necessary calculations and data summaries that a user requires, removing the need to use other software packages such as Microsoft Excel. In this application note, we present a simplified approach for matrix factor determination using the onboard fludics of the Xevo TQ-S and UNIFI Software.
|
LC system: |
ACQUITY UPLC |
|
LC column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 50 mm |
|
Flow rate: |
600 μL/min |
|
Column temp.: |
45 °C |
|
Mobile Phase A: |
0.1% Formic acid |
|
Mobile Phase B: |
Acetonitrile |
|
Gradient: |
5% B to 95% B over 2 min |
|
MS system: |
Xevo TQ-S |
|
|
MS/MS parameters: |
Transitions:Clopidogrel 322.1 > 212.1 d4-clopidogrel 326.1 > 216.1 |
|
|
Ionization: |
Positive ESI |
|
|
Capillary voltage: |
1.00 kV |
|
|
Collision energies: |
16 eV |
|
|
Cone voltage: |
35 V |
UNIFI Scientific Information System
Three lots of hemolyzed plasma were prepared by adding the appropriate volume of hemolyzed whole blood (human, K2EDTA) to plasma (human, K2EDTA) resulting in 5%, 10%, and 15% hemolysis (i.e., 50 μL of hemolyzed blood was combined with 950 μL of plasma to yield 5% hemolyzed plasma). In addition to these three lots, non-hemolyzed blank plasma was also used in the matrix factor evaluation. Each lot of matrix was extracted in replicates of six using a protein precipitation extraction technique where 100 μL of the appropriate matrix was precipitated with 300 μL of methanol, vortex mixed and then centrifuged. Three levels of clopidogrel/ d4-clopidogrel infusion solutions were prepared in methanol at the following concentrations: 10 ng/mL, 50 ng/mL, and 100 ng/mL.
Using UNIFI Software, specific analysis types were defined whereby the method automatically selects specific settings, parameters, and calculations that characterize the analysis type. For example, the post-column infusion MF analysis type is set up such that the software will calculate matrix factor based on the specific sample types entered by the user.
In the sample list, the user inputs the solution injections as “blank,” and the matrix extracts as “matrix spike,” shown in Figure 1. If there are multiple concentrations to be used for MF determination (e.g., low, mid, and high concentrations), these are defined as levels for both the blank and matrix spike sample types.
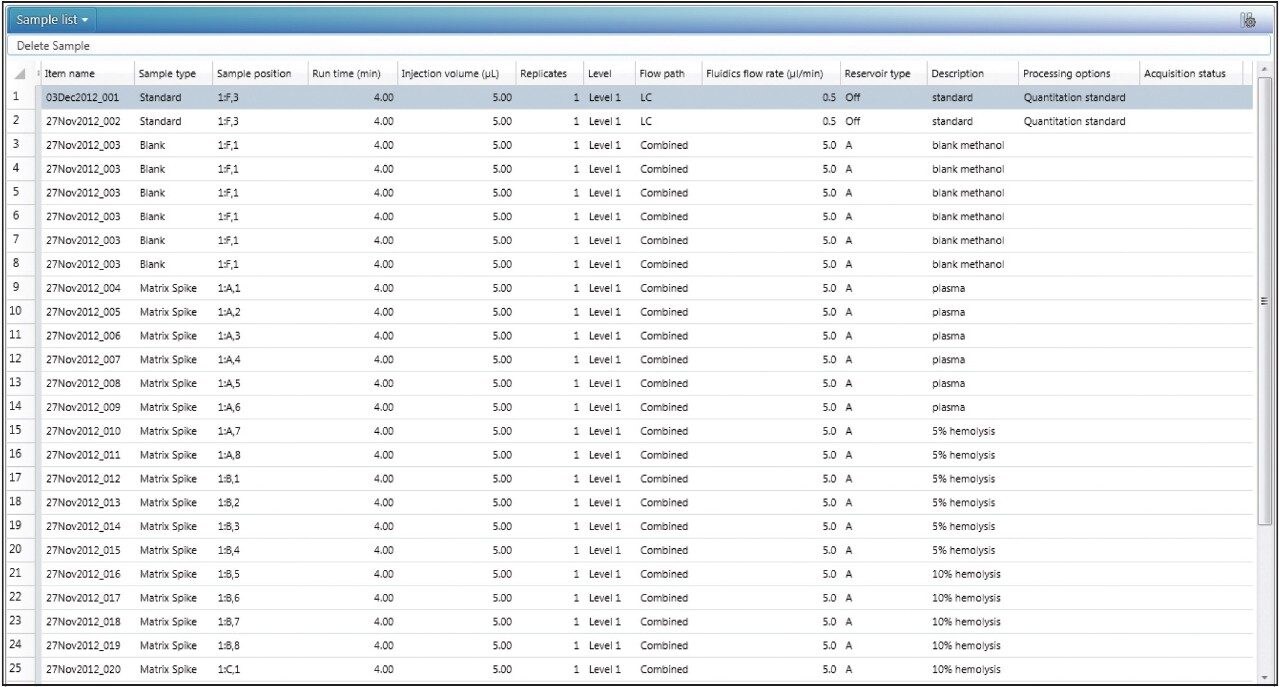
Once the data has been acquired and processed, the MFs will automatically be calculated based on the summary calculations built into the MF analysis type. There is no longer a need to use additional software, such as Microsoft Excel, to calculate and summarize the MF values.
By simply choosing “matrix factor results” on UNIFI’s Review tab, the calculated matrix factor data is displayed on a per-component basis, shown in Figure 2 with calculated statistics such as mean, standard deviation, and relative standard deviation (or coefficient of variation). In addition, the user can view chromatograms and summary plots within the same window.
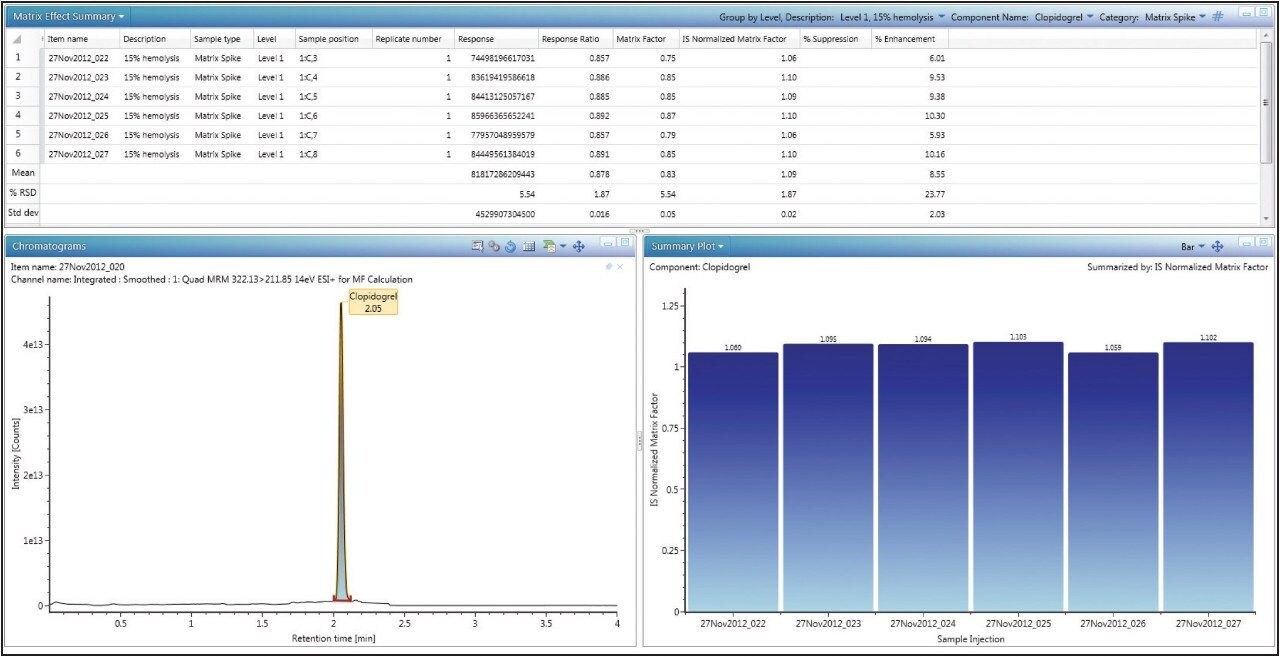
The resulting MFs for all lots of hemolyzed and non-hemolyzed matrix prepared at each concentration level are displayed in Table 1. A matrix factor with a value of less than one indicates suppression, a value greater than one indicates enhancement, and a value of one indicates there is no effect of the matrix on the analyte signal.
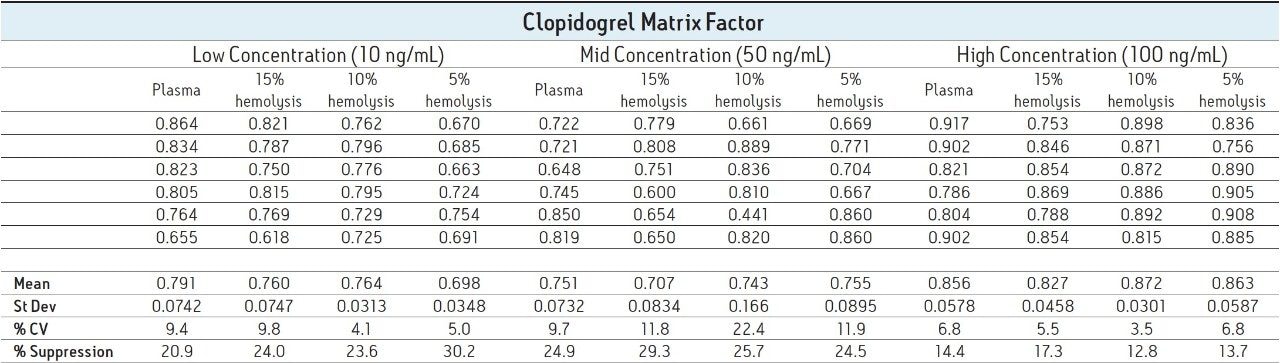
The data shows that at each concentration level, there is no discernible variation between the different lots of matrix, therefore the varying degrees of hemolysis are not impacting the produced signal. The obtained MF values for clopidogrel do show some variation between the different concentrations, with suppression values ranging from approximately 12% to 30% across all injections. It is not surprising that suppression is seen in this assay given that the extraction technique used was a protein precipitation, which provides relatively little clean-up to the samples.
In a bioanalytical assay, it is preferable that a deuterated version of the analyte be used as the internal standard (IS) since it will behave in the same manner chromatographically and spectroscopically as the analyte of interest. This includes ion suppression/enhancement since the analyte signal and the IS signal should be impacted in the same way and to the same extent. It is for this reason that matrix factor is often reported as IS normalized matrix factor to take this fact into account. IS normalized matrix factor is defined as the matrix factor of the analyte divided by the matrix factor of the internal standard. Table 2 reports the IS normalized matrix factor for the four lots of matrix.
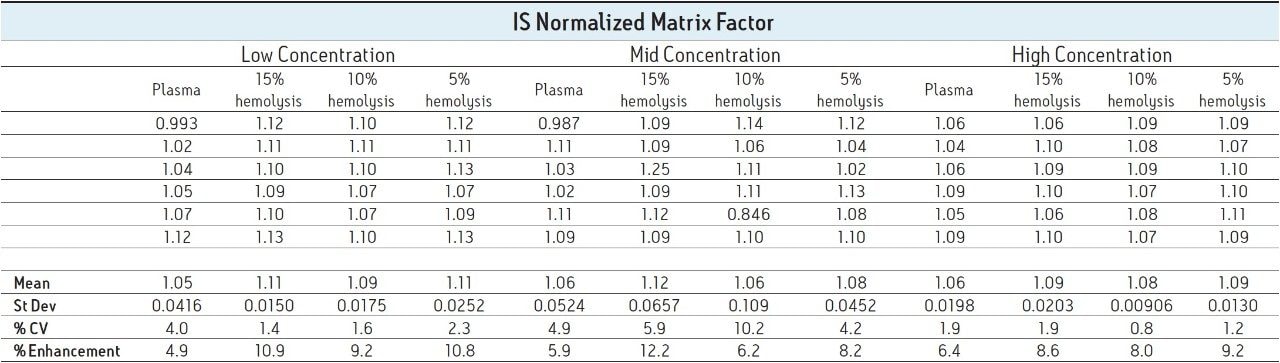
As shown, the IS normalized values are all relatively close to one, indicating that the internal standard was suppressed to the same degree as the analyte; thus, when normalized, negligible suppression/enhancement is seen.
720004627, March 2013