
In this application note, we report the development of a highly sensitive solid phase extraction, and LC-MS/MS assay using the Xevo TQD for the analysis of the pioglitazone and the two active metabolites in human plasma with an assay sensitivity of 10 pg/mL.
Waters Oasis micro-elution plates, ACQUITY UPLC H-Class System, and an advanced tandem quadrupole mass spectrometer (Xevo TQD) were used for the development of a sensitive method for quantification of pioglitazone in human plasma. This application note addresses some critical challenges in the world of bioanalysis – developing a robust and reproducible method to quantify small molecules with the desired sensitivity.
Pioglitazone is part of the thiazolidinedione class of drugs used in the treatment of diabetes through hypoglycemic action. It selectively stimulates the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) to modulate the transcription of the insulin-sensitive genes involved in the control of glucose and lipid metabolism.1
Following oral administration ranging from 15 to 45 mg, the dosed compound undergoes hepatic metabolism – with CYP2C8, and to a lesser degree CYP3A4, to give rise to two active metabolites; keto pioglitazone and hydroxy pioglitazone. Both metabolites are present at higher systemic concentrations than the parent compound at steady state, which is reached seven days after dosing. At steadystate, in patients with type 2 diabetes, pioglitazone comprises approximately 30% to 50% of the peak total pioglitazone serum concentrations (pioglitazone plus active metabolites) and 20% to 25% of the total AUC.
In this application note, we report the development of a highly sensitive solid phase extraction, and LC-MS/MS assay using the Xevo TQD for the analysis of the pioglitazone and the two active metabolites in human plasma with an assay sensitivity of 10 pg/mL.
Samples were prepared using an Oasis solid phase HLB μElution solid phase extraction plate. The plasma samples, measuring 300 μL, were mixed with 20 μL of internal standard solution (deuturated analogues of all three compounds) and 300 μL of 2% phosphoric acid. The samples were applied to the solid phase extraction plate, which had previously been conditioned and equilibrated with methanol (200 μL) and water (200 μL). The sample was washed with a 5% methanol/water solution, then eluted with a 50-μL then 25-μL aliquot of methanol. Samples were further diluted with 75 μL of water, prior to injection.
The analysis was performed on an ACQUITY UPLC H-Class System. A 10-μL aliquot of the sample was injected onto an ACQUITY BEH C18 2.1 x 50 mm, 1.7 μm column. The column was operated under gradient conditions over 2 minutes at a flow rate of 600 μL/min. Mobile phases used were 0.1% ammonium hydroxide and methanol. The column effluent was monitored using a Xevo TQD Mass Spectrometer operated in multiple reaction monitoring (MRM) positive ion electrospray mode.
The transitions monitored were:
Pioglitazone: 357 > 134
Keto pioglitazone: 371 > 148
Hydroxy pioglitazone: 373 > 150
d4-pioglitazone: 361 > 138
d4-keto pioglitazone: 375 > 152
d5-hydroxy pioglitazone: 378 > 154
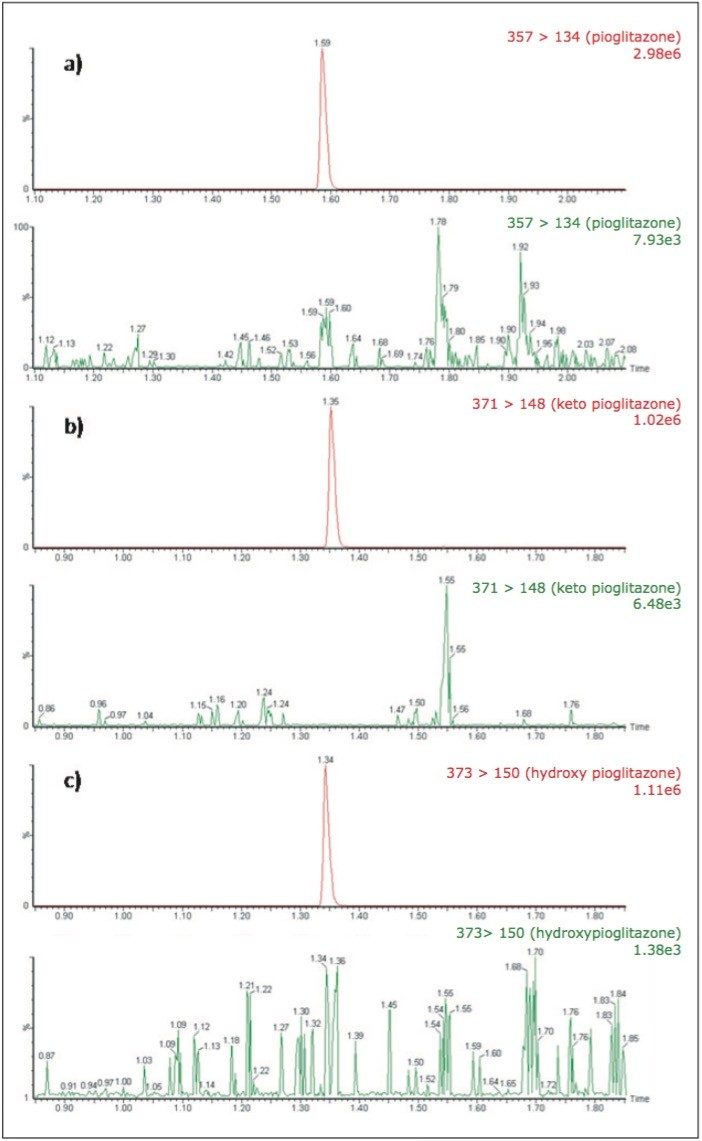
Pioglitazone, keto pioglitazone, and hydroxy pioglitazone eluted with retention times of 1.59, 1.35, and 1.34 minutes, respectively, as shown in Figure 2. This data shows the peaks produced by the chromatography system are very symmetrical, and have a width at the base of approximately 3 seconds for all three compounds. The narrow peak width, and the symmetrical nature allow for efficient processing and peak integration. The data displayed in Figure 2 illustrates the injection of an extracted plasma blank injection, immediately following analysis of the 1000 pg/mL standard. We can see from this data that there is no discernable carryover in the blank chromatogram (in which the baseline has been magnified) for any of the compounds. The extremely low carryover exhibited by the ACQUITY UPLC H-Class System allows the full sensitivity of the Xevo TQD Mass Spectrometer to be exploited.
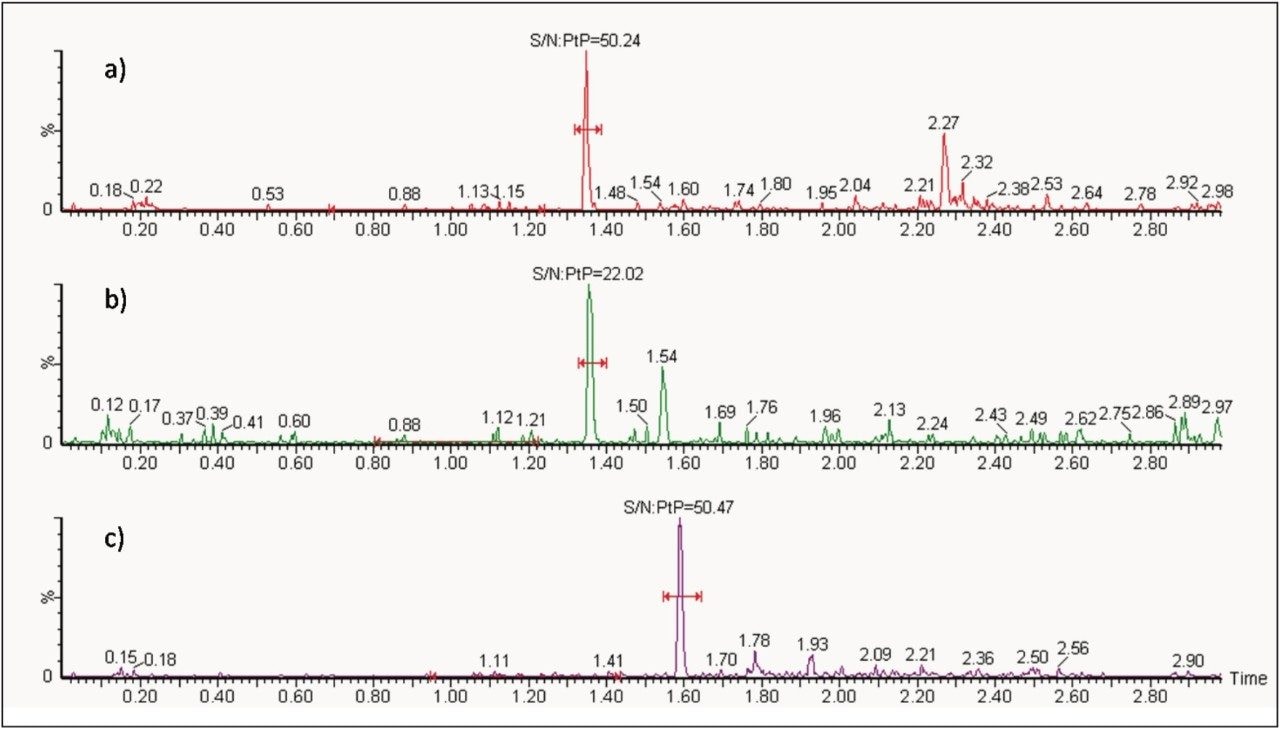
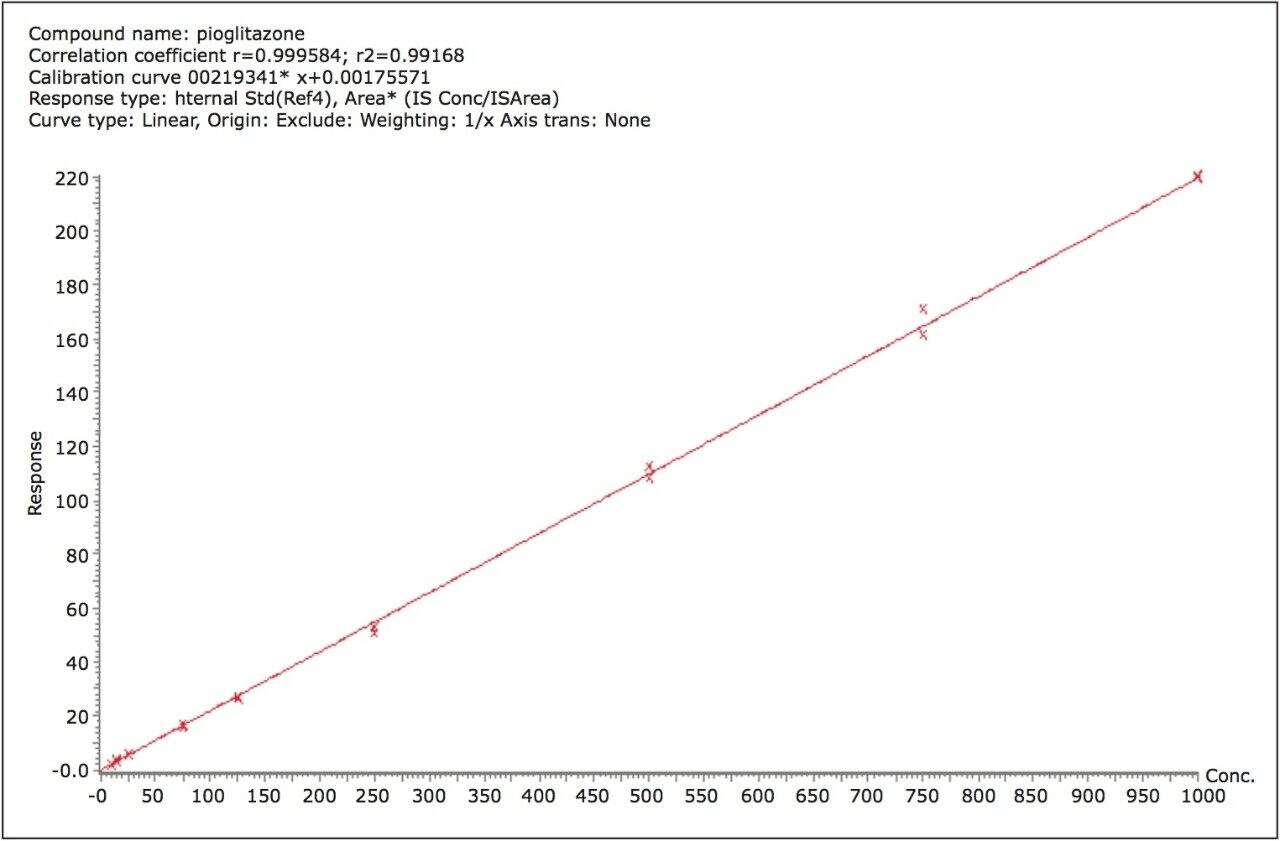
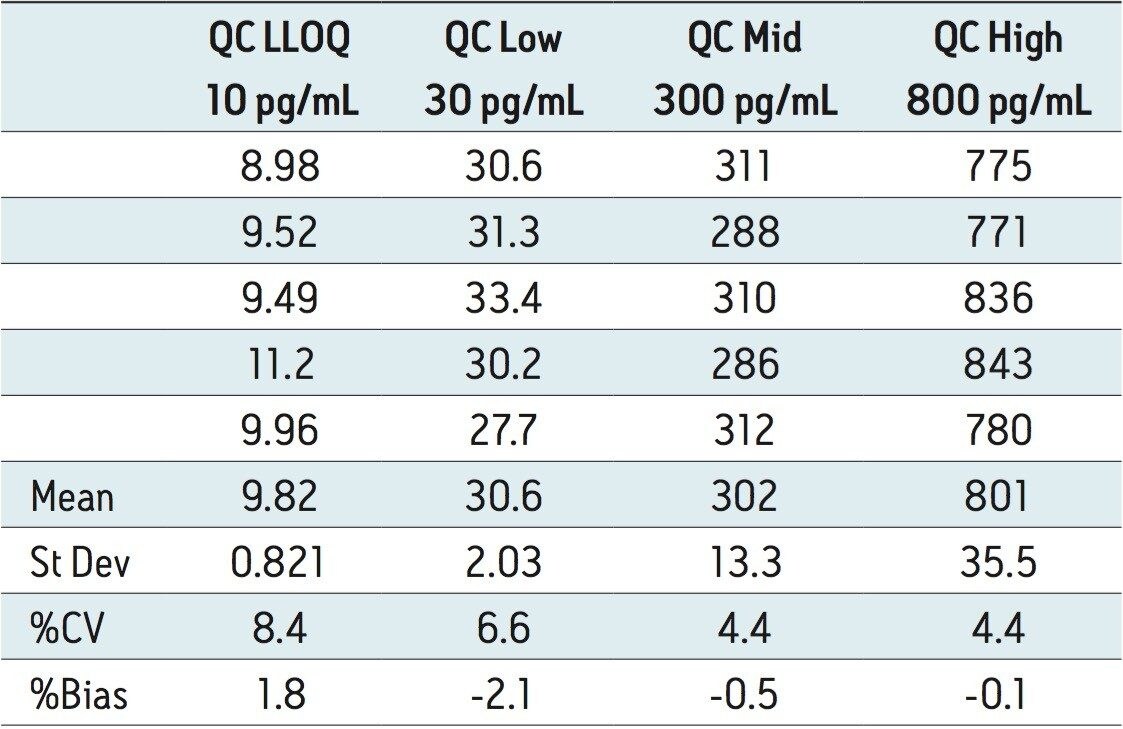
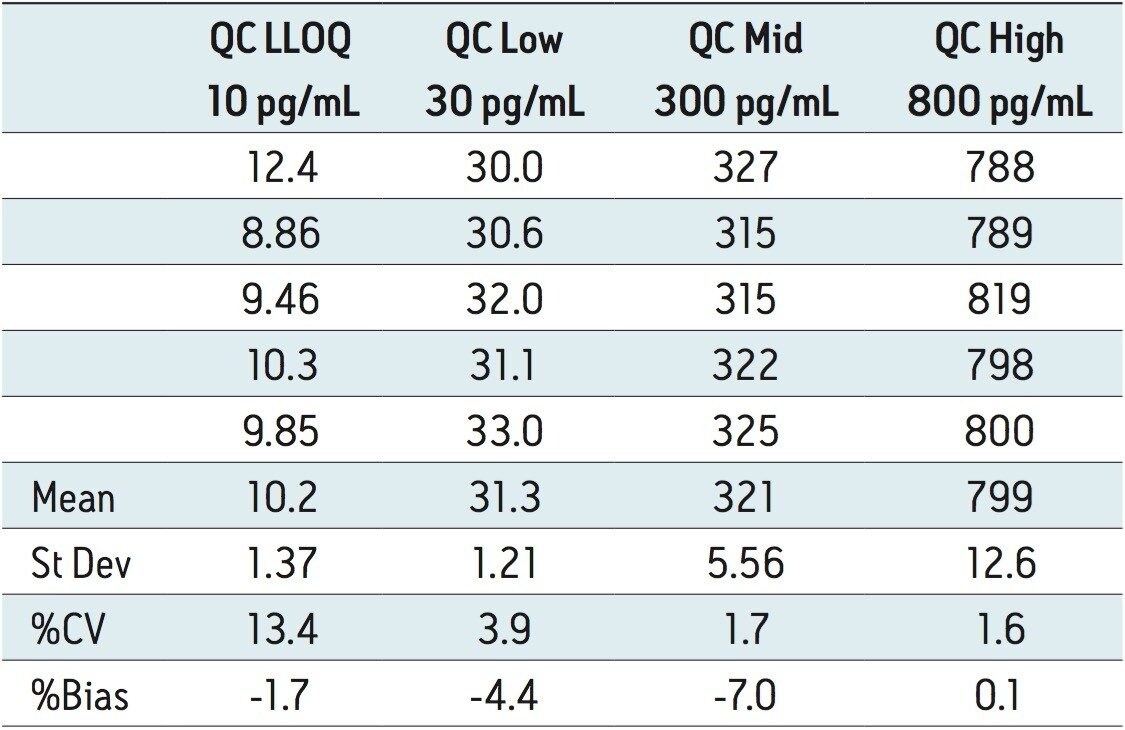
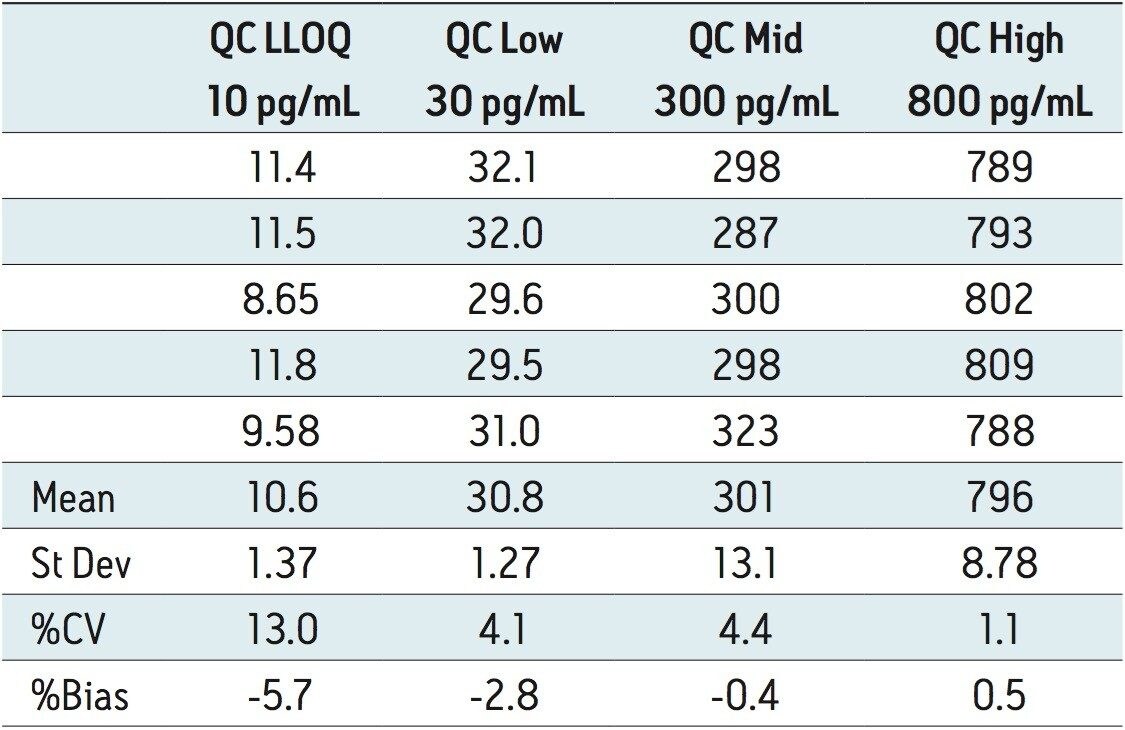
The lower limit of quantification (LLOQ) for the assay was 10 pg/mL for all three analytes. Calculated signal-tonoise values for the LLOQ were 50:1 pioglitazone/ hydroxy pioglitazone, and 22:1 keto pioglitazone, as shown in Figure 3. A typical calibration curve obtained for the assay of pioglitazone is shown in Figure 4. The correlation coefficient ranged between 0.997 and 0.999 for the three compounds using a 1/x weighting linear regression. The single-day accuracy and precision data is displayed in Tables 1 through 3 for quality control (QC) samples prepared at four levels, spanning the calibration range, and extracted in replicates of 5. The validation data shows that the coefficient of variation for the parent and two metabolites ranged from 8.4% to 13.4% for the 10 pg/mL LLOQ with a bias between -5.7% and 1.8%. For the high QC (800 pg/mL), the coefficient of variation ranged from 1.1% to 4.4% with a bias between -0.1% to 0.5%.
720004422, July 2012