This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to detect non-targeted contaminants in infant formula while acquiring multiple MRM data for routine quantitation.
Xevo TQ MS with RADAR functionality was used to detect melamine contamination during routine quantitation of vitamins in infant formula.
Infant formulae and follow-on formulae are heavily regulated throughout the world. These regulations stipulate the allowable amounts of nutritional ingredients and provide specific levels for certain contaminants, such as pesticides that might be used in ingredient production.
However, accidental or intentional contamination can take place at any stage during the manufacturing of infant formulae. An example of this occurred in China in 2008, when thousands of babies were affected by melamine contamination of milk used in the production of infant formulae.1 Unexpected and unregulated contaminants, such as melamine in 2008, are not included in routine product analyses by infant formulae manufacturers. This means that potentially dangerous contaminants can remain undetected until their harmful effects are noticed.
Manufacturers would benefit from technology that can simultaneously acquire both routine quantitative data and related data for untargeted contaminants in the background matrix. These data would be available for retrospective review to identify any potential contaminant species. This could serve as an “early-warning” mechanism, after which the analyst would carry out further tests to confirm the presence of the suspected harmful compound.
Waters Xevo TQ MS, coupled with ACQUITY UPLC, was used for the routine analysis and quantitation of water-soluble vitamins in a sample of infant formula prepared from powder. Prior to analysis, the prepared infant formula was spiked with 1 ppm (1 mg/kg) melamine solution. The Xevo TQ MS was operated in the RADAR acquisition mode. This allowed for the simultaneous acquisition of water-soluble vitamin multiple reaction monitoring (MRM) data and full scan MS data, without compromising the quality of the quantitative data. Figure 1 shows all of the chromatograms for the water-soluble vitamin MRM data (A to I), along with the full scan MS total ion chromatogram (TIC) data (J).
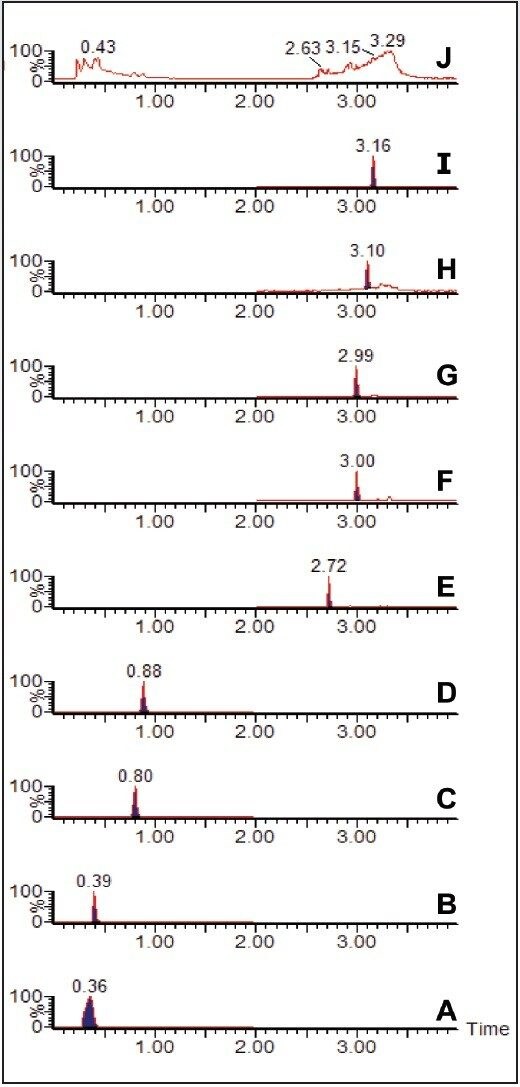
The full scan data can be interrogated, and extracted ion chromatograms (XICs) can be generated for suspected contaminant compounds. Figure 2 shows the XIC for the mass of protonated melamine, m/z 127. The inset in Figure 2 shows the background subtracted spectrum for the peak at 0.34 minutes. The [M+H]+ ion for melamine is clearly seen.
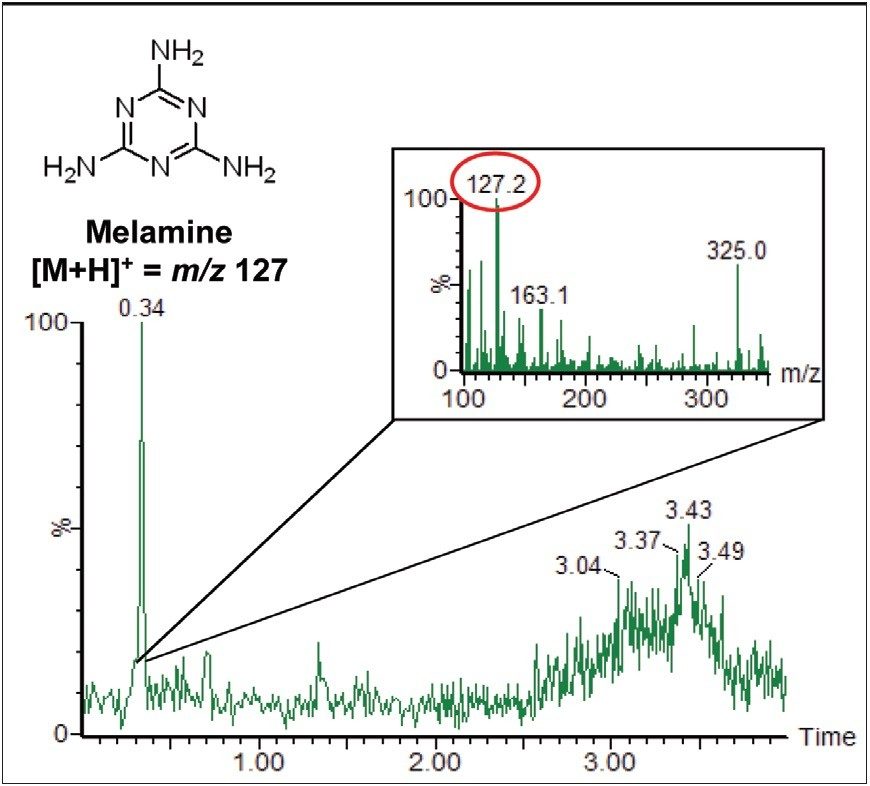
ACQUITY UPLC separation and detection by Xevo TQ MS using RADAR functionality, was successfully used to distinguish a background contamination ion while simultaneously analyzing and quantifying water-soluble vitamins in infant formula. The RADAR full scan data did not compromise the quality of the MRM quantitation data, and the melamine contamination was easily observed in both the XIC and associated spectrum.
This approach offers manufacturers a rapid, easy, and reliable way of both acquiring their tandem quadrupole routine quantitation data, and for monitoring the matrix background for possible unexpected contaminants, in a single injection. Interrogating historical full scan data could help to identify potential product contamination before it reaches the customer. Extremely costly product recalls could be avoided, and injury to both the consumer and the manufacturer’s reputation could be prevented.
720003806, November 2010