This is an Application Brief and does not contain a detailed Experimental section.
In this application brief, we test the analytical reproducibility of the ACQUITY UPLC H-Class System in applications that require long shallow gradients to resolve complex mixtures, such as peptide mapping.
The ACQUIT Y UPLC H-Class System provides reproducible and accurate control of peptide mapping separations over extended series of runs.
Peptide mapping is used to confirm the primary structure of a protein, identify post-translational modification (PTM), and analyze potential impurities. Any difference in the structure of a protein should be reflected in a change in retention time for the peptide containing the modification. The relative amount of the peptide with and without a particular modification is used to measure the fraction of the protein in the particular sample that carries that modification.
Changes in area proportions correspond to the fraction of the protein molecules in the sample having a particular modification.
To meet these application requirements, long, shallow gradients are required. In the past, such separation conditions have been regarded as challenging for single-pump gradient systems. The ACQUITY UPLC H-Class System was tested with a typical peptide mapping protocol.
The ACQUITY UPLC H-Class System consisted of the Quaternary Solvent Manager (QSM), Flow-Through-Needle Sample Manager (SM-FTN), Column Heater, and Photodiode Array (PDA) Detector. The optional 250 µL mixer was installed. The MassPREP BSA Digestion Standard was separated on a Peptide Separation Technology ACQUITY UPLC BEH 300 C18 Column. A shallow gradient of 1.0% per column volume, about 0.6% per minute, was selected as typical of peptide mapping gradients.
The protocol takes advantage of the Auto•Blend capabilities of the instrument. Reservoirs of pure solvents and stocks of concentrated modifiers are used in place of binary, pre-formatted solvent. In this example, a gradient is formed between pure water and pure acetonitrile in lines A and B, while a fraction of the flow is drawn from reservoir D that contains 1% TFA in water. During the gradient, the percentage from line D decreases from 5% to 4%, corresponding to 0.05% to 0.04% TFA, to minimize baseline drift.
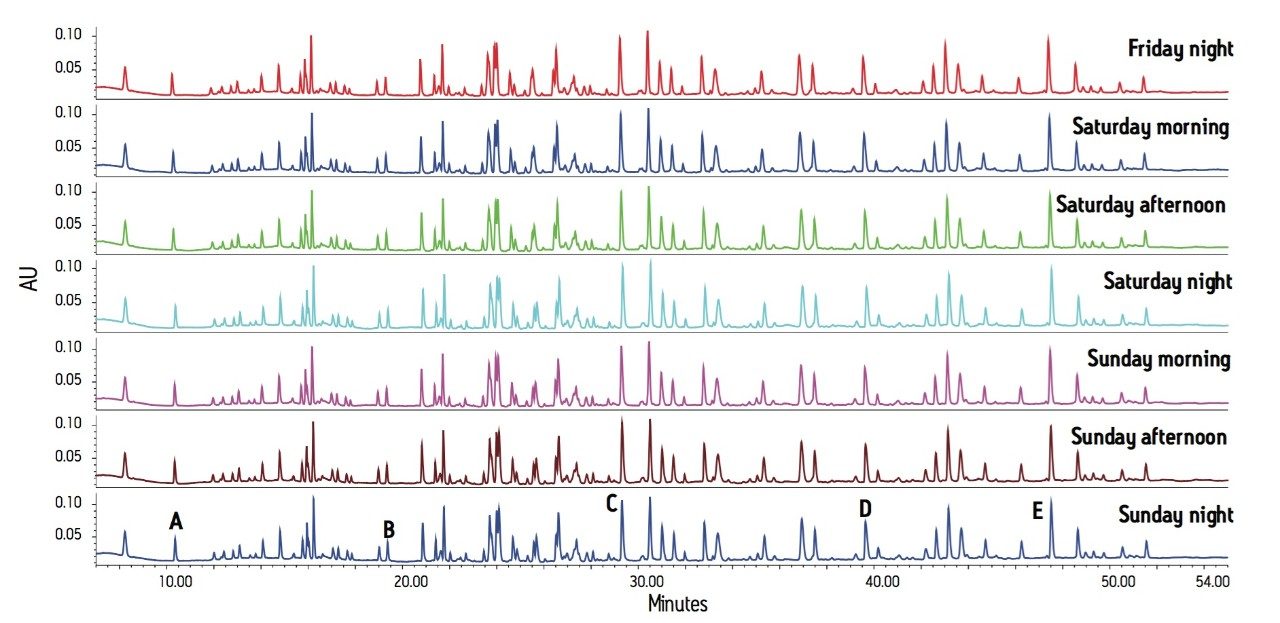
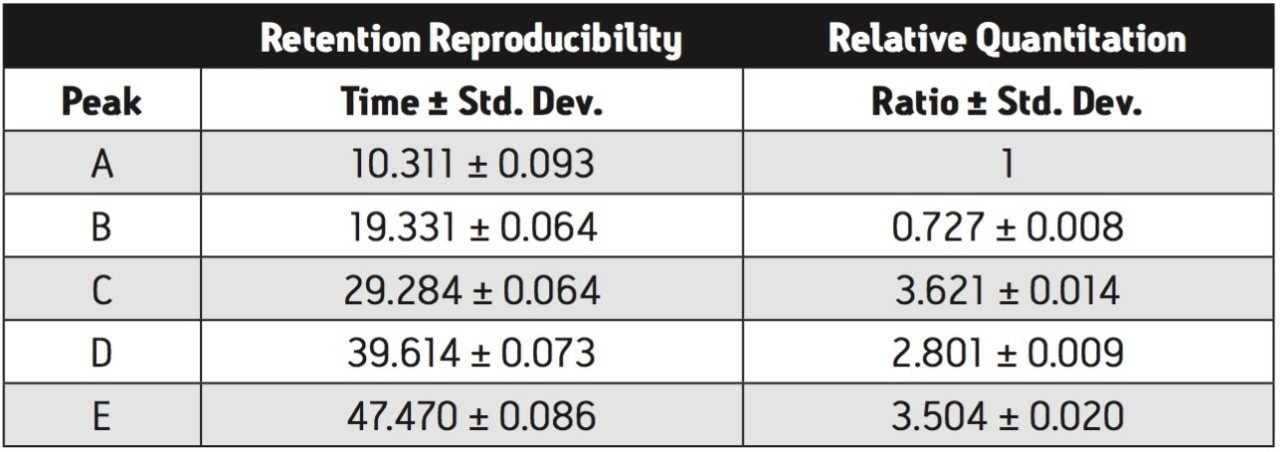
The peptide maps are shown in Figure 1 with both retention time and peak area statistics summarized in Table 1. Relative retention and resolution remain constant over this long series of runs. The retention times are sufficiently reproducible as to ensure that peaks will always be correctly identified. Peak area ratios were calculated comparing peaks B, C, D, and E to peak A. The consistency of these area ratios (Table 1) meets the requirements for estimating proportion of modified protein in a set of samples.
For meaningful peptide mapping, both quantitative and qualitative reproducibility are required. The ACQUITY UPLC H-Class System provides precise control of peptide mapping separations over large sample sets, so that the analyst can be confident that any deviation in retention time indicates a change in sample composition rather than instrument variability. The observed separations meet this objective while taking advantage of the multi-solvent blending capability. The system has been designed to ensure that both qualitative and quantitative results meet the requirements of modern analytical biochemistry.
720003288, January 2010