
This application note describes the use of Waters Xevo TQ MS for the high sensitivity determinations of dexamethasone, at the European MRL level in food. In addition, the use of PICS acquisitions for further presence confirmation is explored.
Ensuring consumer safety is a priority for governments, international regulatory bodies and organizations that process and handle products prior to consumption. Food safety issues arising from commodity products often become globally reported and have the potential to impact consumer confidence and trade at international levels.
Dexamethasone and betamethasone are synthetic glucocorticoids widely used in animal husbandry1. These epimeric compounds are licensed for therapy in veterinary practice, while their use as growth promoters is banned within the European Union (corticosteroids are listed in Annex I of European Council 96/23 - group B2f)2.
In order to protect consumer safety, Maximum Residue Limits (MRLs) have been fixed for both molecules by the European Community in several matrices, for instance: 0.3 μg/L (ppb) in bovine milk and 2.0 μg/kg (ppb) in liver from different species3.
The major challenge in the analysis of dexamethasone and betamethasone consists of performing an efficient separation of both epimers and detecting and identifying these molecules at the required maximum residue limit (MRL). Malone et al.4 and Li et al.5 have illustrated efficient methods for the separation of both epimers.
This application note describes the use of Waters Xevo TQ MS for the high sensitivity determinations of dexamethasone, at the European MRL level in food. In addition, the use of PICS acquisitions for further presence confirmation is explored.
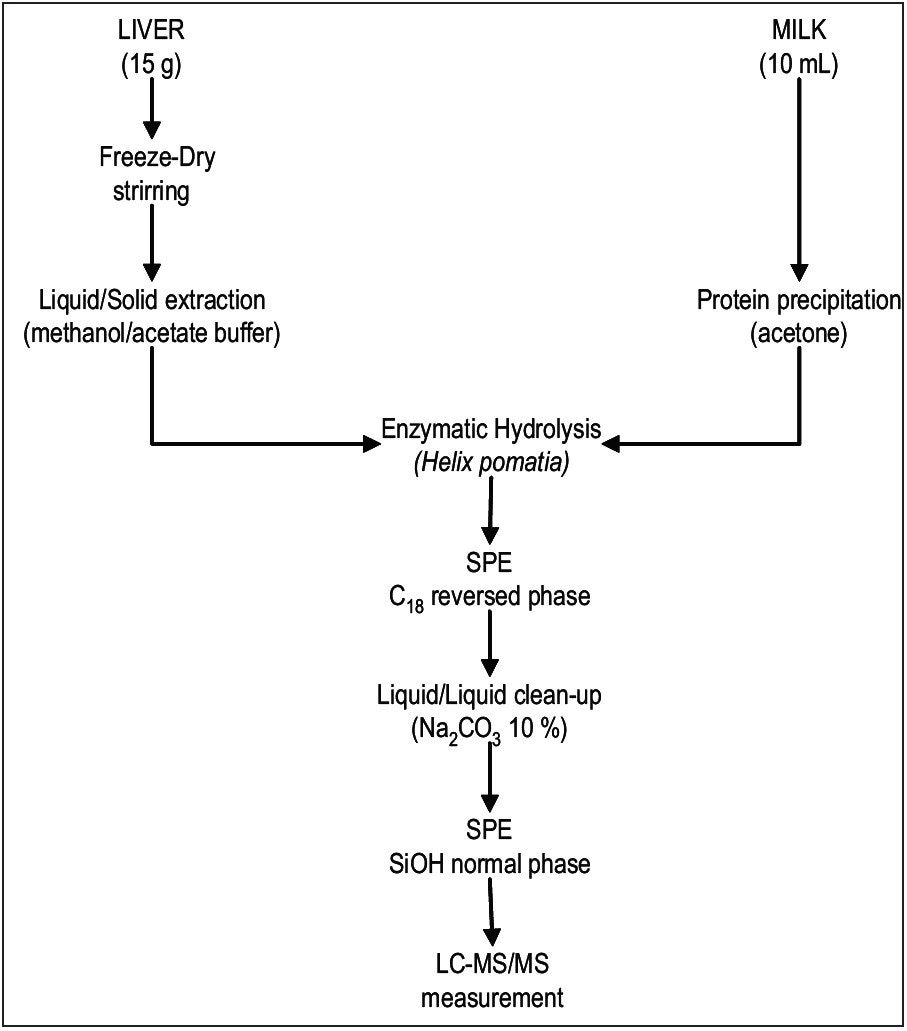
Sample extraction and purification procedure in liver is described in detail elsewhere6. Initial sample preparation for milk included a protein precipitation step which then followed the same procedure as liver (Figure 1).
|
LC system: |
ACQUITY UPLC System |
|
Runtime: |
7.0 min |
|
Column: |
ACQUITY UPLC BEH C18 Column 1.7 μm, 2.1 x 100 mm |
|
Mobile phase A: |
0.5% acetic acid dissolved in water |
|
Mobile phase B: |
acetonitrile |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
2 μL |
|
MS system: |
Xevo TQ MS |
|
Ionization mode: |
ESI negative |
|
Capillary voltage: |
3 kV |
|
Source temp: |
150 °C |
|
Desolvation temp: |
500 °C |
|
Desolvation gas: |
920 L/H |
|
Collision gas flow: |
0.15 mL/min |
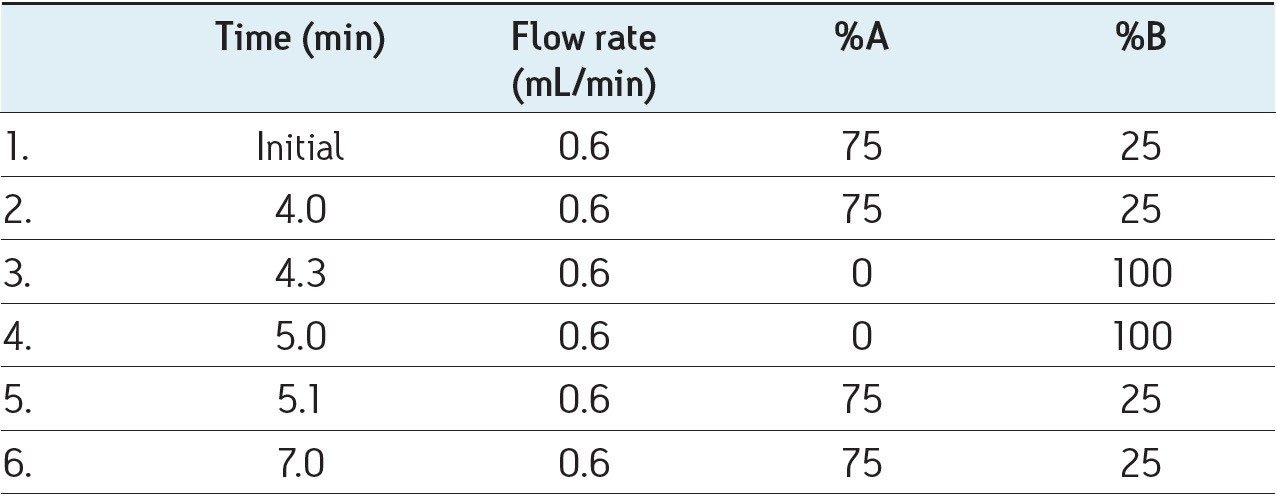
Diagnostic MRM transitions were first generated using Waters’ IntelliStart Technology. All the parameters (detailed in the following table) were then optimized individually for each diagnostic signal.
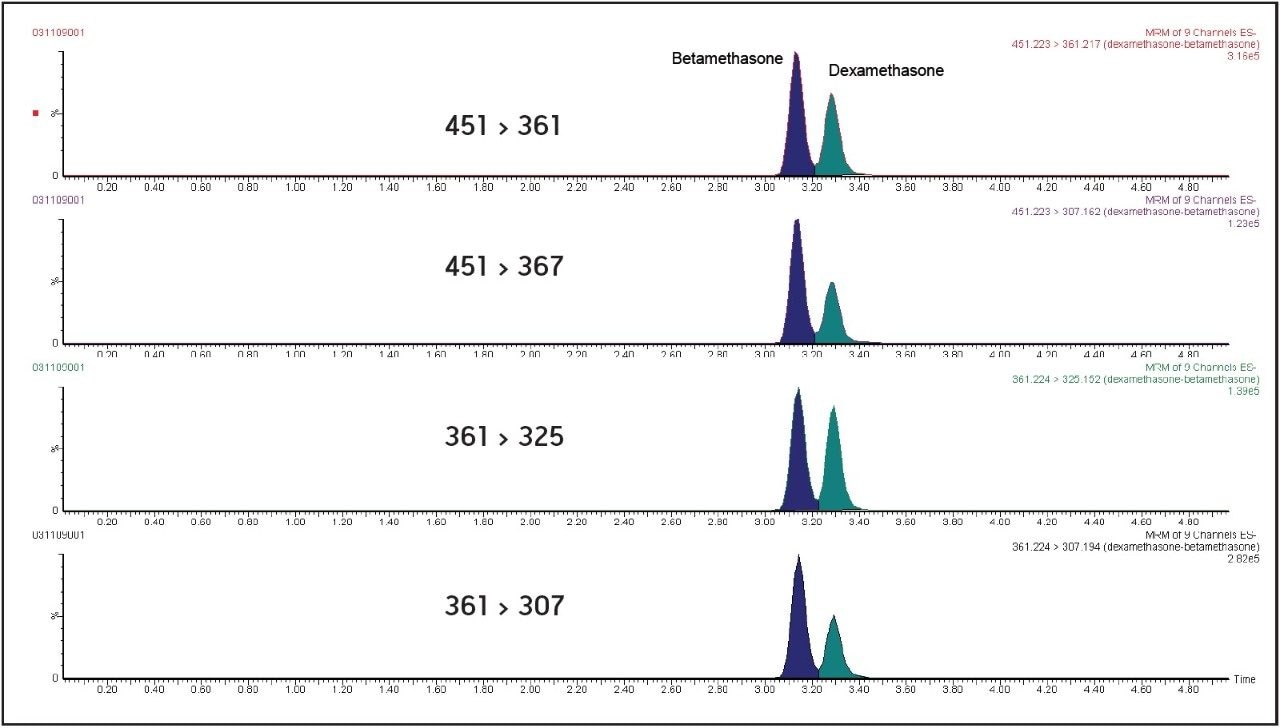
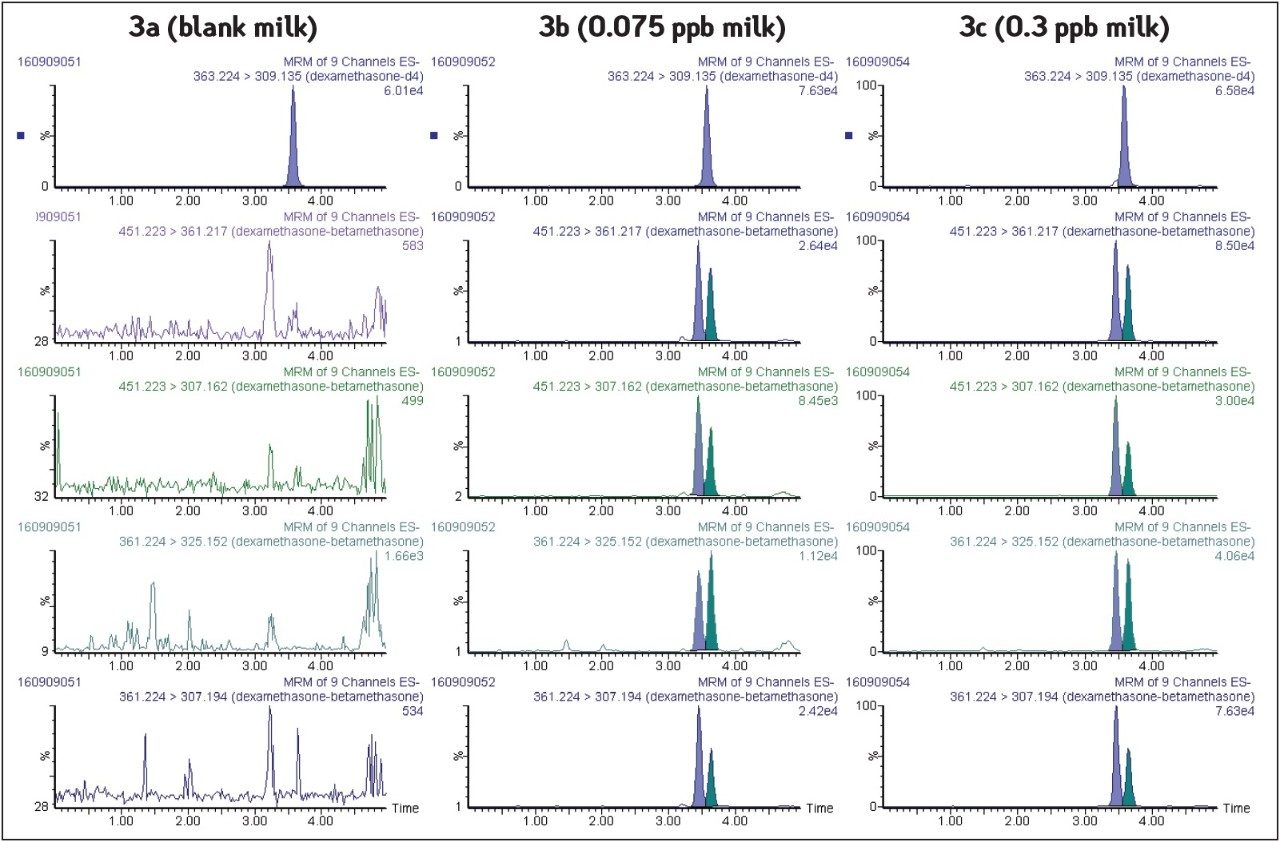
In order to obtain accurate determinations for these particular growth promoters, it is essential that chromatographic separation of the epimers dexamethasone and betamethasone is achieved. An efficient separation of betamethasone (tR=3.14 min) from dexamethasone (tR=3.25 min) was observed using isocratic gradient in the range [0-4 min]. These UPLC conditions allow identification of molecules from their relative retention time (Figure 1).
Four diagnostic MRM transitions were set for the identification of dexamethasone and betamethasone in order to fulfil the regulatory requirements of the European Commission Decision 2002/657:EC7: these four diagnostic transitions (451 > 361, 361 > 307, 451 > 307, and 361 > 325) were selected in the MRM transition mode in order to perform unambiguous identification of the compounds. Moreover, d4-dexamethasone (363 > 309, tR= 3.56 min) was used as internal standard because of its mimetic properties with dexamethasone.
Blank milk sample chromatograms are shown in Figure 3a with no positive response for dexamethasone and betamethasone at the expected retention time. This demonstrates the selectivity of the methodology with the combination of chromatographic resolution and instrumental selectivity. Extracted MRM chromatograms corresponding to milk samples fortified at 0.3 μg/L (MRL) and 0.075 μg/L (4 times below MRL) are shown in Figures 3b and 3c. High instrument sensitivity allows highly confident identification of both substances to be performed (Identification points=10), even at concentrations that are 4 times below the European MRL.
To test performance around the regulatory limits, fortified liver samples were analyzed at twenty times below, four times below, and at the European MRL for dexamethasone (2.0 ppb). The extracted MRM chromatograms for each level are shown in Figure 4. Positive identification according to regulatory requirements (2002/657/EC) was comfortably achieved with 10 identification points (IP=10) for concentrations around the MRL (3 IPs are mandatory with 4 IPs reserved for illegal substances). As with previous determinations in milk, the method selectivity was such that the blank liver samples did not show any response (were s/n > 3).
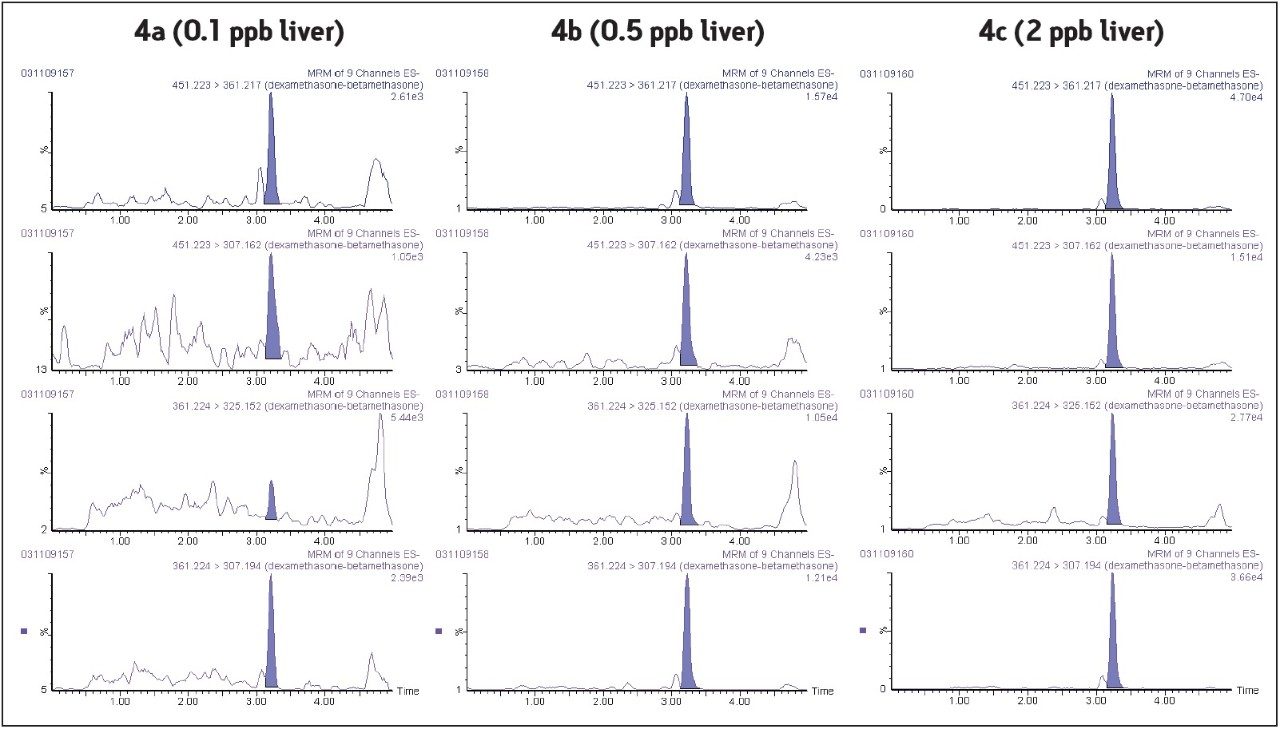
The stability of the transition ratios was also evaluated in the different matrices for all concentrations. Signals obtained were very repeatable even at very low concentration levels (MRL/4 for milk: 0.075 ppb and MRL/20 for liver: 0.1 ppb) with an unambiguous identification of the compound (IP=10).
Concerning the liver matrix, linearity was evaluated by plotting relative peak height ratios in the range [0 to 10 μg/kg]. The correlation coefficients of the calibration curve were above 0.99. At the MRL level, the relative standard deviations for signal responses were calculated to 6.4% and 6.1% for betamethasone and dexamethasone. At the same level, the relative standard deviations for relative retention times were 0.16% and 0.20% for betamethasone and dexamethasone respectively.
CCα (decision limit) and CCβ (detection limit) were determined in accordance with European Decision 2002/657. CCα were 2.31 and 2.35 while CCβ were 2.57 and 2.63 for dexamethasone and betamethasone respectively.
Data directed acquisition allows spectral data to be collected when a particular parameter is detected above a certain threshold. Very rapid switching between quadrupole static mode and scanning mode is essential for this to happen in real time whilst a chromatographic peak is eluting. This capability allows additional information when trying to confirm presence of residues in a sample and is an additional step that can be taken to help identify and investigate false positive results.
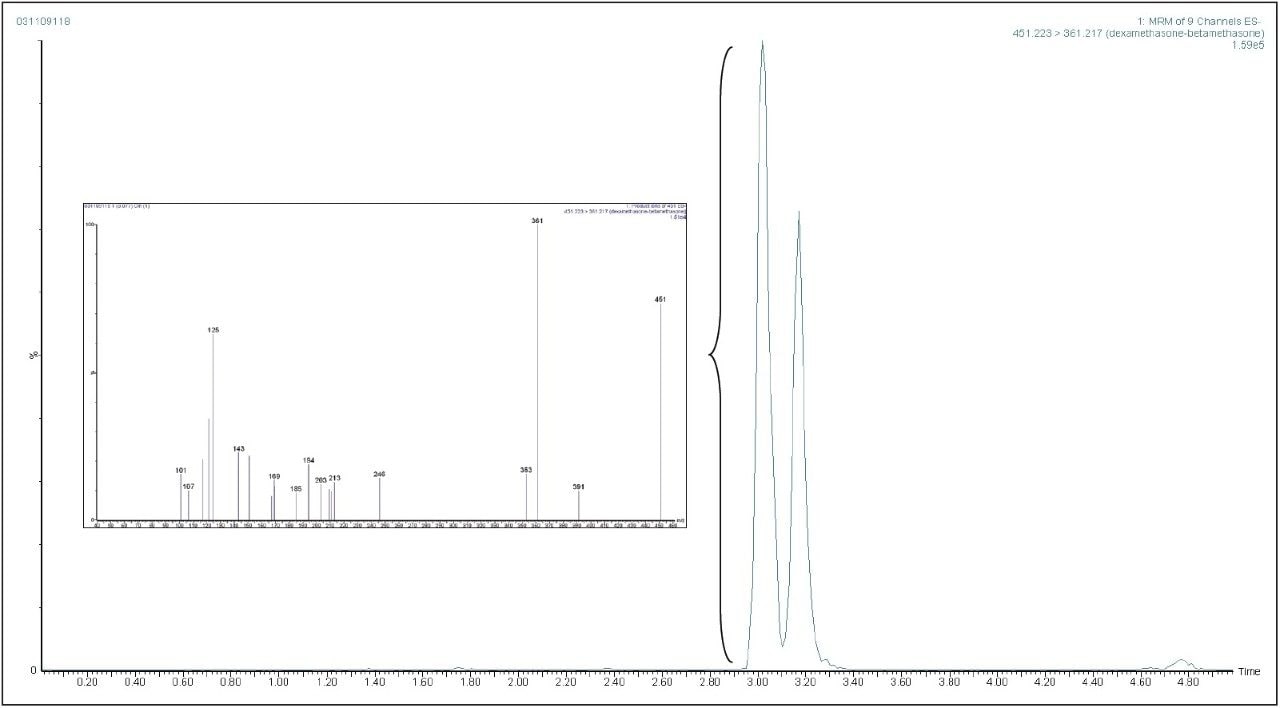
Product ion confirmation scan (PICS) allows a product ion scan to be acquired which is triggered by a selected MRM transition reaching a critical threshold. This capability is especially relevant when applied to supporting the identification of a target substance. Figure 5 shows extracted MRM chromatogram for dexamethasone at European MRL in a liver sample which has PICS enabled. Figure 5 also shows the product ion spectrum from the automatically triggered scan from the 361 > 307 transition. This spectrum can then be compared to an MS/MS library to provide additional evidence for presence of dexamethasone.
720003378, April 2010