
This application note describes a simple, rapid approach to bioanalytical method development using UPLC and Tandem Quadrupole MS.
The accurate determination of the concentration of drugs in biological fluids is essential to define the pharmacokinetic parameters in animals or human and requires a robust, reliable, analytical method. Additionally, this methodology must possess sufficient sensitivity to characterize the elimination phase of a drug and the pharmacokinetic curve, as well as have adequate specificity to resolve the drug compound from other circulating drugs, endogenous compounds, and metabolites. This was traditionally achieved by LC/UV, with analysis times of 20 to 40 minutes.
The advent of API Tandem Quadrupole MS instrumentation in the early 1990s has allowed LC methods to be dramatically shortened, relying instead on the specificity of the multiple reaction monitoring (MRM) process to effect analyte resolution. The resulting run times of two to five minutes are now commonplace.
Recent U.S. FDA guidelines1 on metabolite interferences and ion suppression have brought the need for good chromatographic resolution back into sharp focus.
The methods development process is a delicate balance between throughput, resolution, and sensitivity, requiring many parameters to be evaluated to obtain the best method. This is a very time-consuming and laborious process requiring a skilled analyst. The advent of Waters UltraPerformance LC (UPLC) technology allows the chromatographic resolution and throughput of sub-2 μm column particles to be utilized in a routine lab environment.
In this application note, we describe a simple, rapid approach to bioanalytical method development using UPLC and Tandem Quadrupole MS.

The common benzodiazepine drug, alprazolam, used to treat anxiety disorders and panic attacks, was analyzed to illustrate our method development process. This compound is rapidly metabolized in the body to the alpha hydroxy metabolite, and is excreted in the urine with a half-life of approximately 16 hours.
Alprazolam and its metabolite were spiked into rat plasma at a concentration of 10 ng/mL, and the plasma was precipitated with acetonitrile (2:1) to represent the most complex case. Chromatographic separations were performed on 2.1 x 50 mm Waters ACQUITY UPLC BEH Columns using C8, C18, Shield RP18, and ACQUITY UPLC HSS C18 chemistries. The columns were eluted with linear gradients using either formic acid (0.1%) or ammonium hydroxide (0.1%) and acetonitrile or methanol.
|
LC system: |
Waters ACQUITY UPLC System |
|
Column: |
Various ACQUITY UPLC Columns, 2.1 x 50 mm |
|
Column temp.: |
40 °C |
|
Flow rate: |
800 μL/min |
|
Mobile phase A: |
Acidic/basic |
|
Mobile phase B: |
Acetonitrile or methanol |
|
Gradient: |
Various, over 2 min |
|
MS system: |
Waters TQ Detector |
|
Ionization mode: |
ESI positive/negative |
|
Capillary voltage: |
1.6 KV |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
800 L/Hr |
|
Source temp.: |
150 °C |
|
Compound |
MRM |
Cone (V) |
Collision Energy(V) |
|---|---|---|---|
|
Alprazolam |
309 > 281 |
50 |
26 |
|
Alprazolam OH |
325 > 297 |
44 |
26 |
|
Alprazolam D5 |
314 > 286 |
50 |
28 |
The development of a sensitive, selective chromatographic methodology requires the judicious selection of the chromatographic column, mobile phase pH, and organic modifier. These factors must be carefully manipulated to produce the required resolution of the component of interest from the endogenous metabolites in the matrix, as these will result in reduced assay sensitivity due to ion suppression. This can be a time-consuming process with multiple combinations of columns, mobile phase pH, and organic modifier to be evaluated.
To fast track the method development process, we have developed a simple strategy to ensure that the optimum methodology is obtained. This approach is based on a reversed-phase separation platform, displayed in Figure 2.
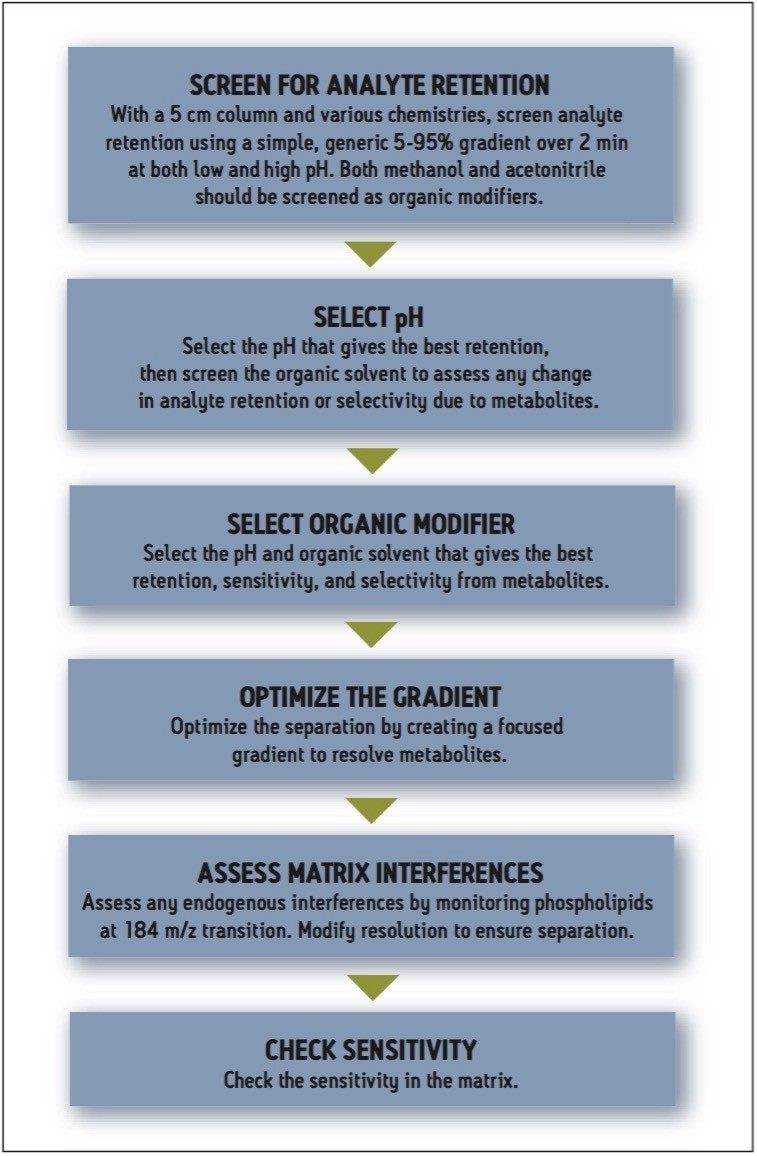
The first step is to screen different column chemistries with low and high pH aqueous mobile phase and acetonitrile or methanol using a simple, generic, 5 to 95% gradient. By employing columns of 5 cm in length, the screening process can be rapidly accomplished using two-minute gradients. From these results, we can evaluate whether sufficient sensitivity can be obtained for the drug compound with any of the combinations of mobile phase pH, organic modifier, and column chemistry. If this is the case, the next step is to optimize the resolution to separate the analyte from any known drug-related metabolites, achieved by manipulating the gradient slope.
Once the analyte and matrix have been satisfactorily resolved, the assay can then be optimized for sensitivity.
The method development approach described here exploits the extra chromatographic efficiency of UPLC and the wide pH stability range (2 to 12 pH) of ACQUITY UPLC BEH chemistries to rapidly develop an effective methodology. Once a suitable mass spectrometry method has been established for the analyte(s) of interest, the column selectivity can be evaluated at low and high pH.
In order to effectively evaluate column selectivity, it is necessary to understand the metabolism of the compound of interest such that coelution of metabolites and the analyte can be avoided.
As an example, the analysis of a typical drug compound (ibuprofen) and its metabolites is shown in Figure 3. Here we can see that there is insufficient resolution between the parent compound and glucuronide metabolite.
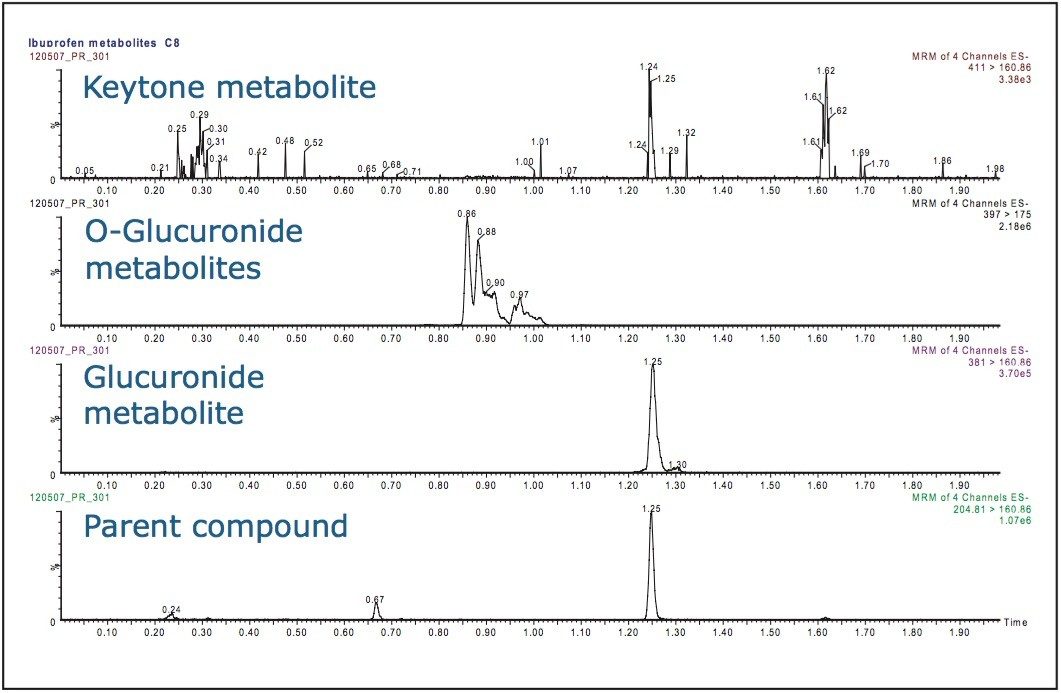
Thus achieving resolution between the analyte of interest and its drug metabolites is critical to ensure that any metabolite that is thermally labile and undergoes decomposition in the source does not revert back to the parent drug and get measured as the parent compound.
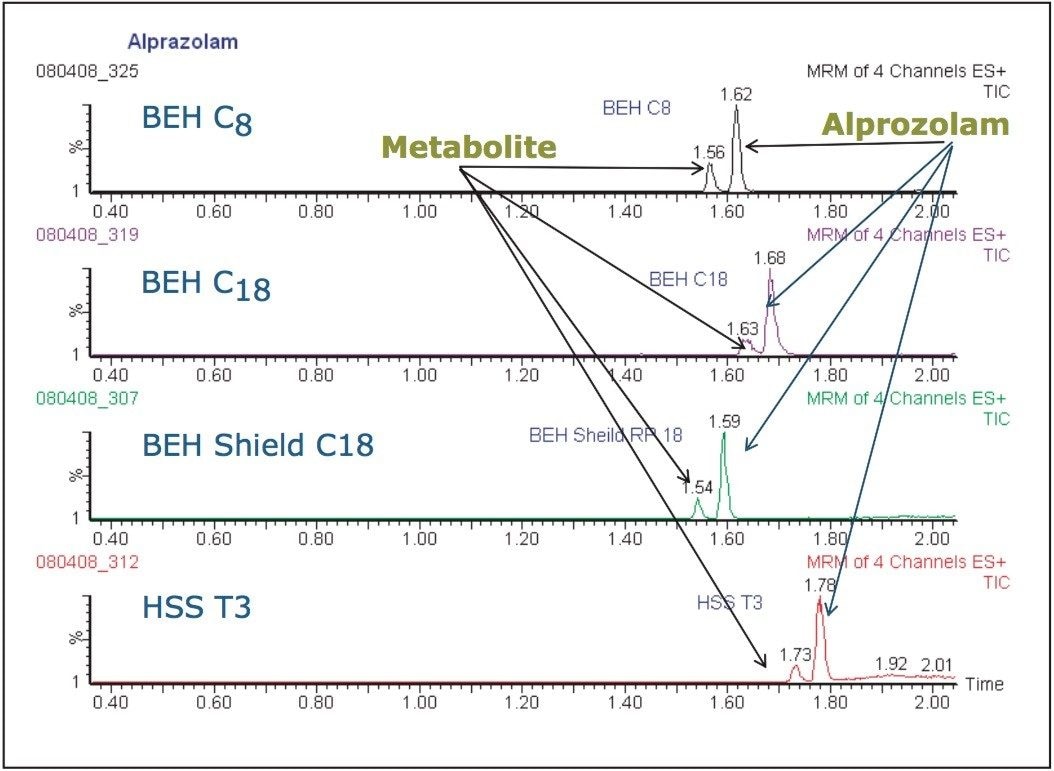
The data displayed in Figure 4 show the separations obtained on the four column chemistries – C18, C8, Phenyl, and High Strength Silica (HSS) – at low pH using formic acid buffer using methanol as the organic modifier. Here we can see that all four columns produced resolution between the two analytes.
From the data in Figure 4, it is clear that all four columns were capable of resolving the metabolite from the analyte. However, the C8 column gave the best overall peak shape.
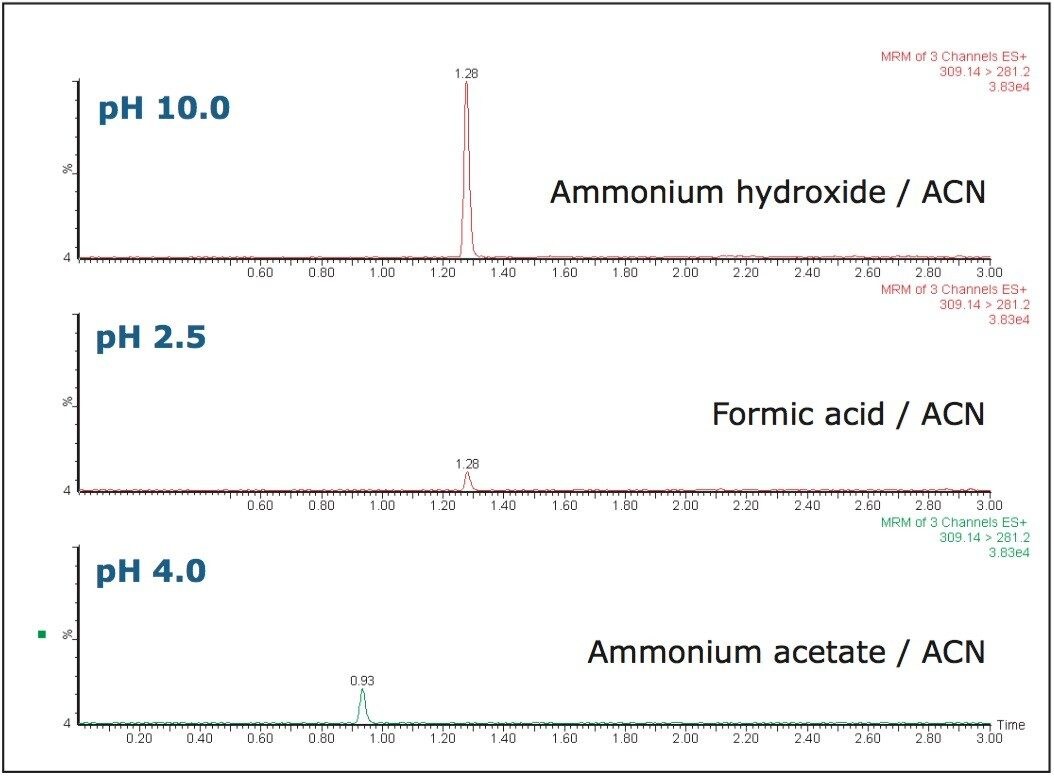
The ability to manipulate the pH of the mobile phase allows the ionization state of the analytes to be manipulated. In a reversed-phase separation system, neutral molecules will be retained longer on the column and produce superior peak shapes. This can result in higher MS sensitivity as the eluting peaks will contain organic concentration and desolvate more efficiently.
As we can see from the data in Figure 5, on this occasion the use of higher pH (10) increased the signal intensity of alprazolam by a factor of 10. We can also see that there is a reduction in background noise. Changing the pH did not adversely affect the analyte’s resolution from metabolites or endogenous matrix peaks.
Once the mobile phase pH has been selected, it is necessary to select the organic modifier that gives the best resolution, peak shape, and assay sensitivity. The two most commonly-employed organic modifiers are methanol and acetonitrile.
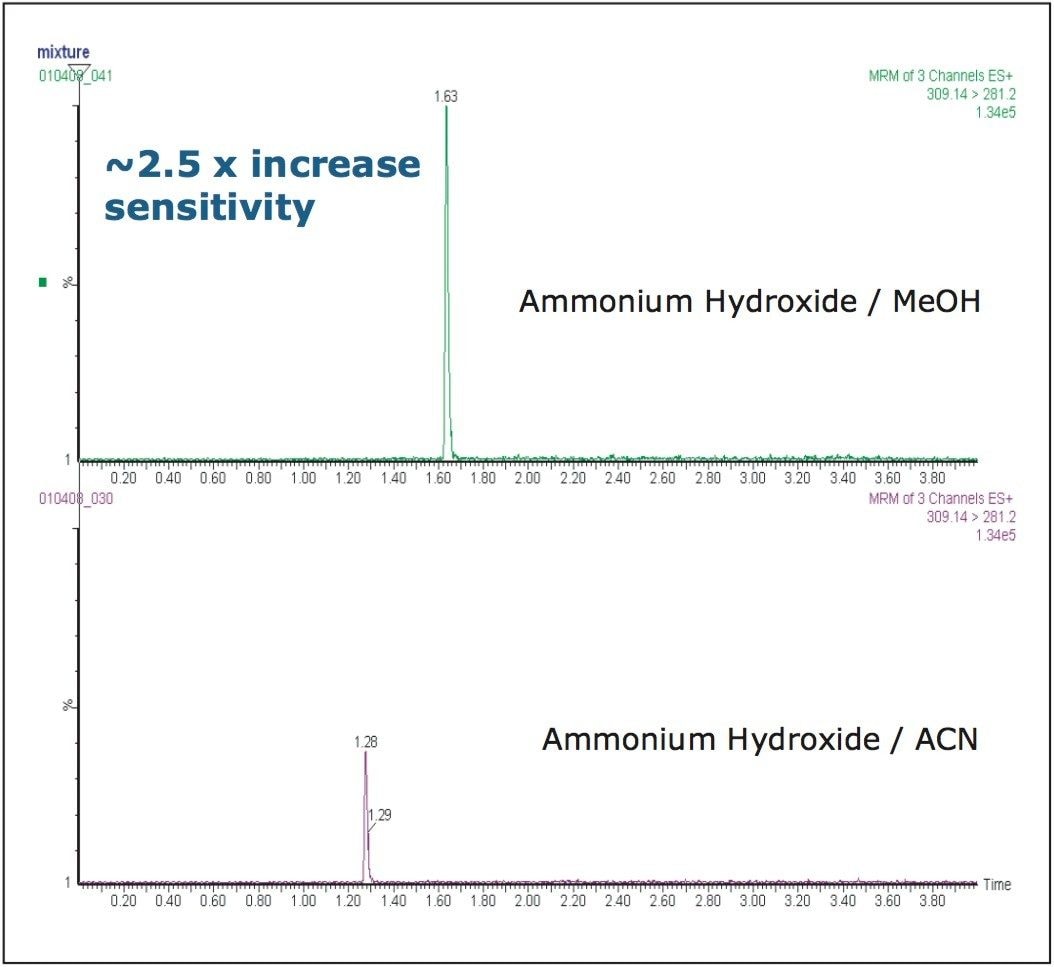
In this example, we evaluated both of these mobile phase modifiers. Results obtained are shown in Figure 6. The data shows that the signal response for the alprazolam molecule is approximately 2.5-fold greater with methanol compared to acetonitrile. As the best resolution, peak shape, and sensitivity were obtained using a high pH aqueous buffer – methanol mobile phase gradient on a C18 column – these conditions were selected for further optimization.
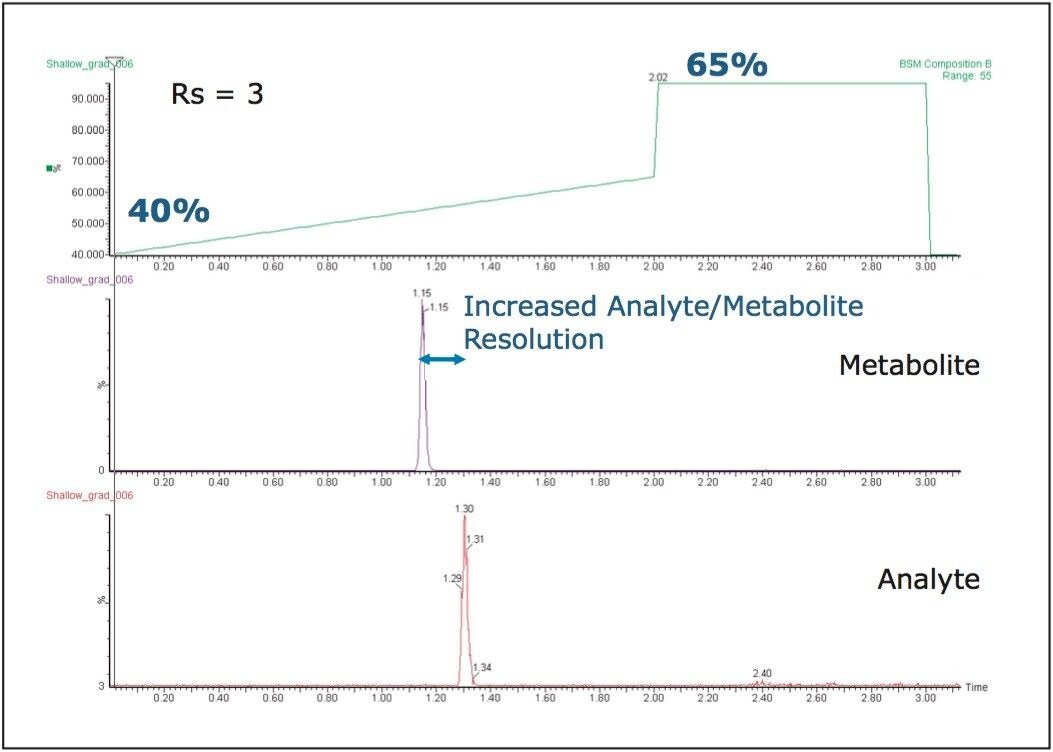
The next step is to adjust the gradient slope to optimize the resolution of the drug from the metabolites and to further decrease the analysis time. To do this, we can take advantage of the fact that metabolites, being more polar than the parent compound, will elute earlier on a reversed-phase system (with the exception of N-oxide metabolites). With the slope gradient reduced and the starting point increased, the resolution also increases while at the same time the required analysis time decreases.
Results shown in Figure 7 demonstrate how changing the gradient slope to 40 to 65% produced an improved analyte/metabolite resolution and a faster analysis time.
While adjusting the gradient profile, it is critical to include a high-strength organic wash to ensure that the column is thoroughly washed prior to injection of the next sample.
In order to ensure a reliable assay, it is critical to ensure that there is no coelution of the analyte of interest with endogenous interferences resulting from the matrix. The primary components in plasma responsible for ion suppression are a class of compounds called phospholipids.
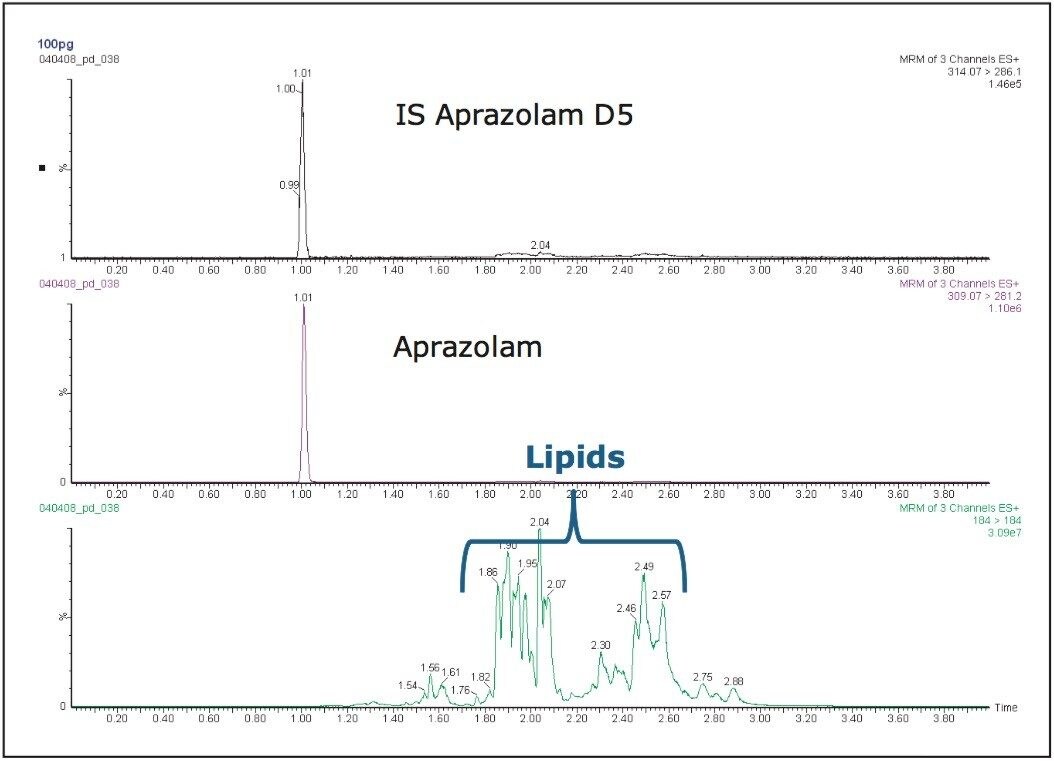
To evaluate whether any coelution with these phospholipids exist, we can use the capabilities of the ACQUITY TQD Mass Spectrometer to obtain both analyte-ion MRM data and lipid product-ion data in the same run. The presence of the phospholipids is monitored using the common fragment ions m/z = 184 and 104 in positive ion mode, while simultaneously monitoring the elution of the drug molecule and its metabolite.
The data displayed in Figure 8 show excellent resolution of the analytes of interest from the endogenous lipids in the sample, and hence would be suitable for the determination of alprazolam in plasma.
The process of bioanalytical method development can be a complicated, time-consuming process often requiring a significant amount of trial-and-error experimentation performed by an experienced analyst. In this application note, we have demonstrated a simple, systematic approach to rapidly develop a high-quality bioanalytical LC method with optimal peak shape, resolution, sensitivity, and speed of analysis.
The high performance and throughput of the ACQUITY UPLC System allowed for the rapid and automated screening of columns, pH, and organic modifier. The mechanical and chemical stability of the ACQUITY UPLC BEH Column stationary phase allowed the complete range of mobile phase pHs to be explored and optimized to maximize MS response.
Further, the extra peak capacity of the UPLC System enabled the complete resolution of the drug, its metabolites, and endogenous metabolites in less than two minutes.
Coupling this system with the ACQUITY TQD Mass Spectrometer, with its fast-scanning capabilities, has enabled the simultaneous measurement of endogenous lipids and analytes of interest with no loss in analyte sensitivity. This MS capability ensures that potential interferences can be monitored continuously over the course of the method development and validation process, increasing confidence in the method’s performance
Waters UPLC-MS/MS technologies combine to provide both robust chromatography as well as high MS sensitivity, in a user-friendly system, that helps ensure that bioanalytical laboratories are performing the quality of analyses that will conform to regulatory guidelines for assessing metabolite interferences and ion suppression.
720002811, September 2008