
Open access software offers the power of chromatography and mass spectrometry to chemists who are not analytical instrumentation specialists. It allows them to quickly and easily know what they've made and allows the experts to work on the difficult analytical problems. An open access UPLC-MS system was investigated for high throughput library QC. In this application note, we describe some of the enhancements to LC and LC-MS technologies that have generated useful tools that improve the throughput and accuracy of these assays.
The identity and purity of a candidate pharmaceutical is critical to the effectiveness of the drug screening process. LC-MS is employed extensively in drug discovery in order to exclude false positives and maintain the high quality of the product. This process can be time consuming and can potentially delay the progression of a drug through the discovery process.
Thus, sample throughput is a critical issue in moving compounds from the hit to lead status. UltraPerformance LC (UPLC) leverages sub-2 μm LC particle technology to generate high efficiency faster separations.
When a photodiode array/evaporative light scattering/mass spectrometry (PDA/ELS/MS) detection scheme is used in conjunction with multiple-mode ionization, the potential for peak detection is greatly improved. Pharmaceutical chemical libraries often contain a great diversity of small molecules to cover a broad range of biological targets.1 In this environment, the ability to obtain information pertaining to multiple MS acquisition modes, in addition to PDA and ELS, in a single injection is invaluable.
Open Access software offers the power of chromatography and mass spectrometry to chemists who are not analytical instrumentation specialists. It allows them to quickly and easily know what they’ve made and allows the experts to work on the difficult analytical problems.
An Open Access UPLC-MS system was investigated for high throughput library QC. In this application note, we describe some of the enhancements to LC and LC-MS technologies that have generated useful tools that improve the throughput and accuracy of these assays.

|
LC system: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18 Column 2.1 x 30 mm, 1.7 μm |
|
Column temp.: |
50 °C |
|
Sample temp.: |
8 °C |
|
Injection volume: |
2 μL |
|
Flow rate: |
800 μL/min |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Gradient: |
5 to 95% B/0.70 min |
|
MS system: |
Waters SQ Detector |
|
Ionization mode: |
E SI positive/ESI negative, multi-mode ionization |
|
Capillary voltage: |
3.0 KV |
|
Cone voltage: |
20 V |
|
Desolvation temp.: |
450 °C |
|
Desolvation gas: |
800 L/Hr |
|
Source temp.: |
150 °C |
|
Acquisition range: |
100 to 1300 m/z |
|
Scan speed: |
2500, 5000, and 10,000 amu/sec |
|
Note: A low volume micro-tee was used to split the flow to the ELS and SQ. |
|
Gain: |
500 |
|
N2 gas pressure: |
50 psi |
|
Drift tube temp.: |
50 psi |
|
Sampling rate: |
20 points/sec |
|
Range: |
210 to 400 nm |
|
Sampling rate: |
20 points/sec |
Maximum efficiency is essential for labs challenged by throughput requirements and the management of data from multiple systems and users. The Waters Open Access suite of software streamlines the integration of analysis with data acquisition, processing, and reporting.
The system and software are initially configured by a system administrator who defines login access, method selection, and reporting schemes. This allows users to analyze their own samples with minimal intervention from analytical support.
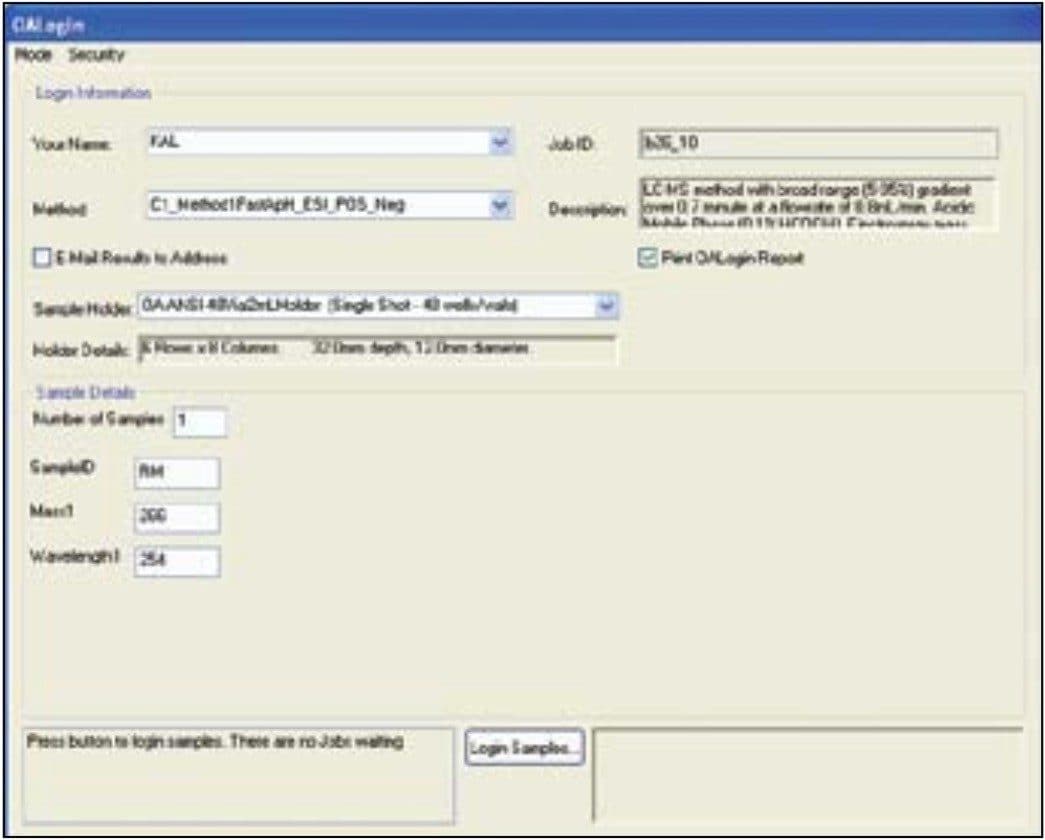
OpenLynx Open Access Application Manager for MassLynx Software is designed to allow chemists to walk up to a terminal and log in samples while entering the minimum information required to run the samples. A series of methods, each including gradient and MS conditions as well as processing parameters, are initially set up by the system administrator. The users choose an appropriate method from the list, importing their sample lists and placing their samples in the position designated by the software. Desired sample analysis is then performed by the configured system. The single page login window can be seen in Figure 2.
Chromatographic separations were carried out using the ACQUITY SQD System coupled to detectors specialized for UPLC separations: the single quadrupole SQ Mass Detector, and PDA and ELS detectors that provided simultaneous signal collection. For additional flexibility, the UPLC system was configured with a Sample Organizer and a Column Manager. The sample capacity of the system totals twenty two 384-well plates, for 8448 library samples in total. This extends the overall walk-away time for the system. The column manager allows four UPLC columns to be installed, heated, and switched into line based on the method requirements. This allows the chemist to take advantage of the broad range of stationary phases that encompass compound types, ranging from very hydrophilic to very lipophilic.
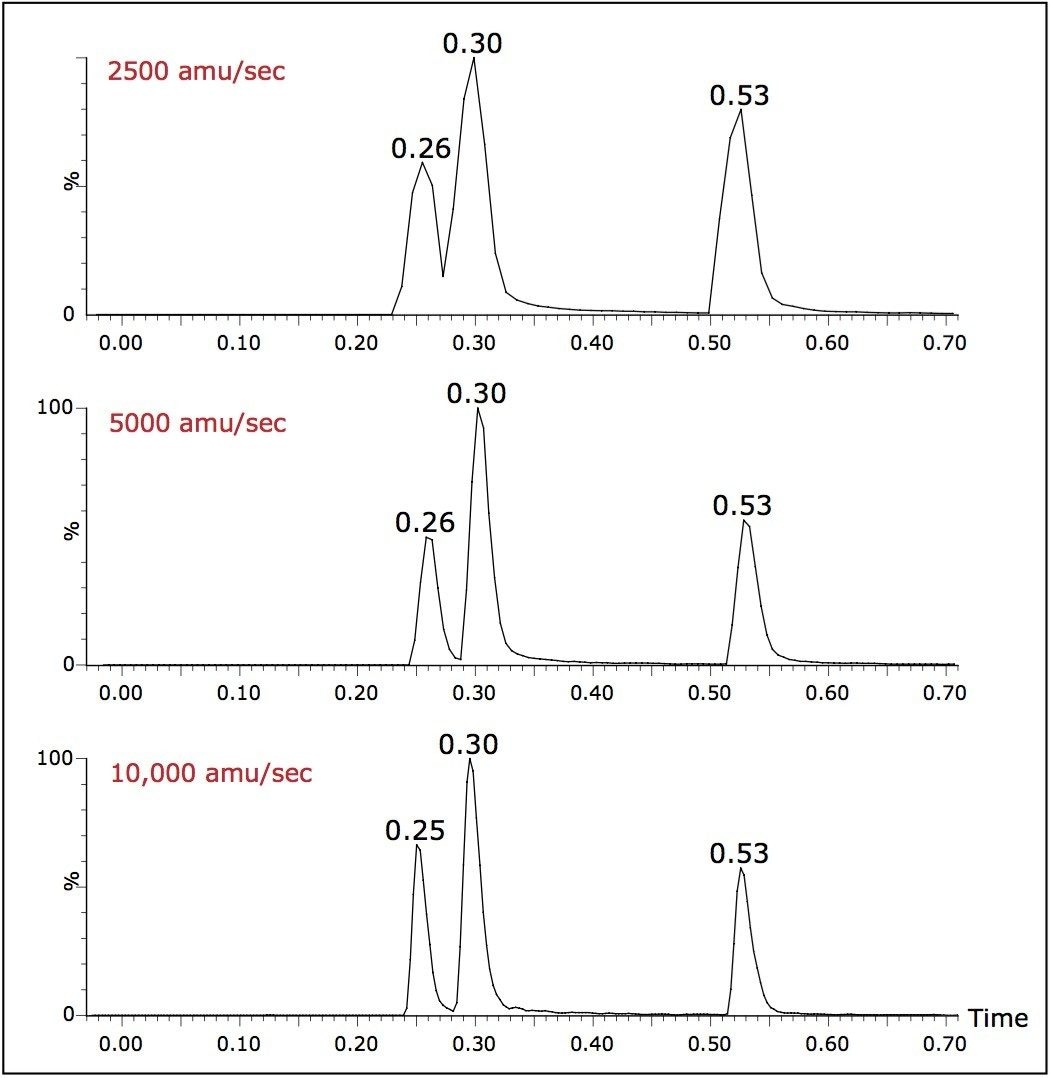
Samples were analyzed using gradients less than one minute in length with a flow rate of 800 μL/min. When analyzing the narrow peaks generated by the UPLC-MS system, the data collection rate can compromise the number of points across the LC peak, resulting in a poor definition of the eluting peak and hence inaccurate results.
The ability of the MS system to collect data at a high scan speed, 10,000 amu/sec, greatly improves chromatographic peak definition. This in turn facilitates the acquisition of a large number of individual acquisition modes in one run while maintaining adequate peak characterization.
As can be seen from the data displayed in Figure 3, the result of operating at lower data collection rates can compromise the chromatographic resolution. To maintain chromatographic integrity, it is therefore advantageous to be able to scan at elevated scan speeds.
The total cycle time of the method was 1 minute 20 seconds, facilitating increased sample throughput while still allowing generous washing steps to prevent sample-to-sample memory effects. Using a flow rate of 800 μL/min and a 2.1 x 30 mm column clears 9 column volumes/min.
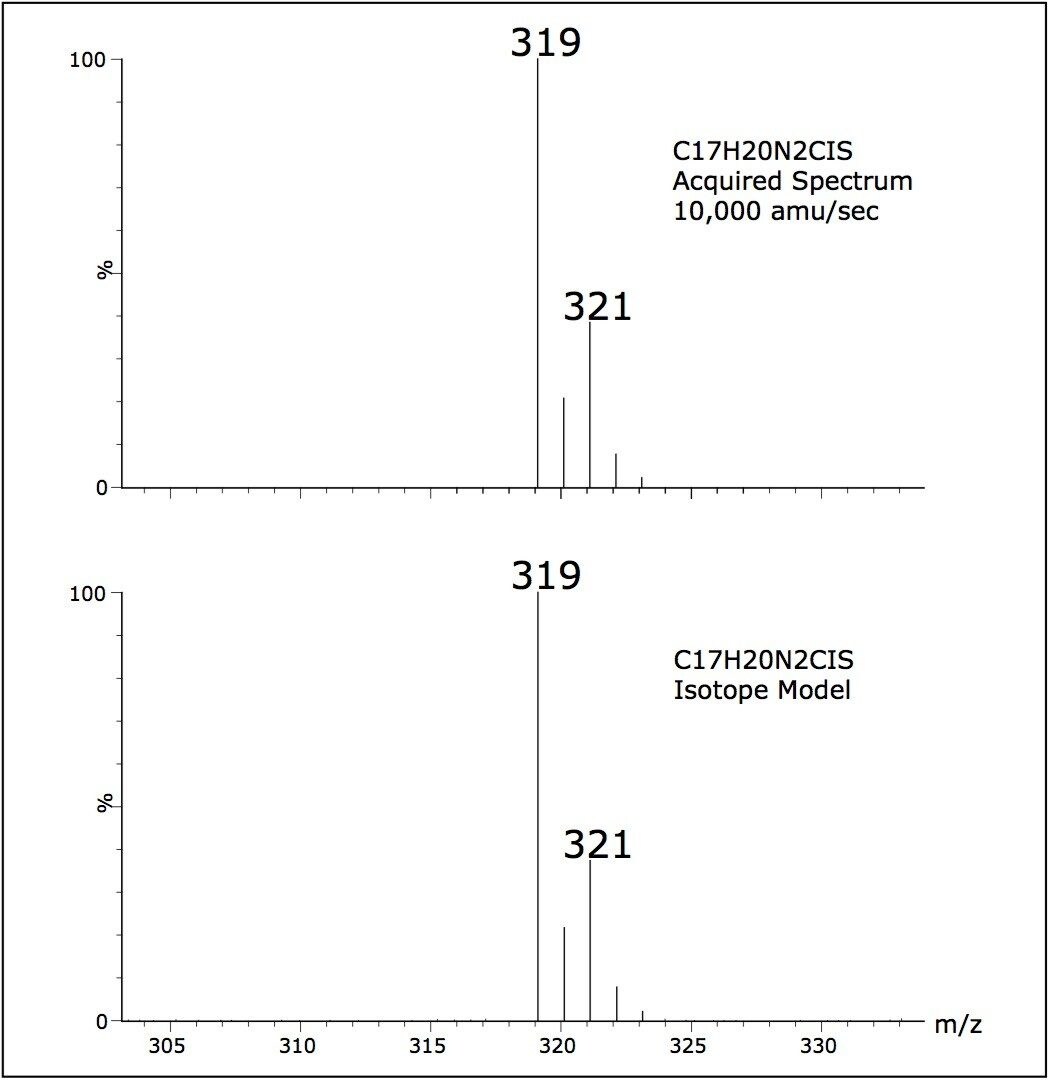
The spectral data quality of scanning experiments carried out from 2500 to 10,000 amu/sec were found to be comparable, thus providing confidence that operating at these rapid data collection rates does not compromise the spectral data quality. Figure 4 shows a comparison of an acquired spectrum with a software generated isotopic model. Isotope ratios of data collected at 10,000 amu/sec were within 1% of the isotopic model, again ensuring data fidelity is not compromised.
In addition to obtaining mass confirmation by multiple MS modes, it is possible to add PDA and ELS detectors to obtain auxiliary information. A single run can then provide UV spectral information and an estimation of compound purity at low wavelengths.
ELS detection is an alternative to UV detection, and does not depend on the presence of a chromaphore. ELS detection works by measuring the light scattered from the solid solute particles remaining after nebulization and evaporation of the mobile phase. Chromatograms illustrating the use of triple detection (PDA/ELS/MS) are shown in Figure 5. The signal from an ELS detector can give a tentative estimation on the relative quantities of the components present. It has been known to give rise to similar responses for related compounds.2
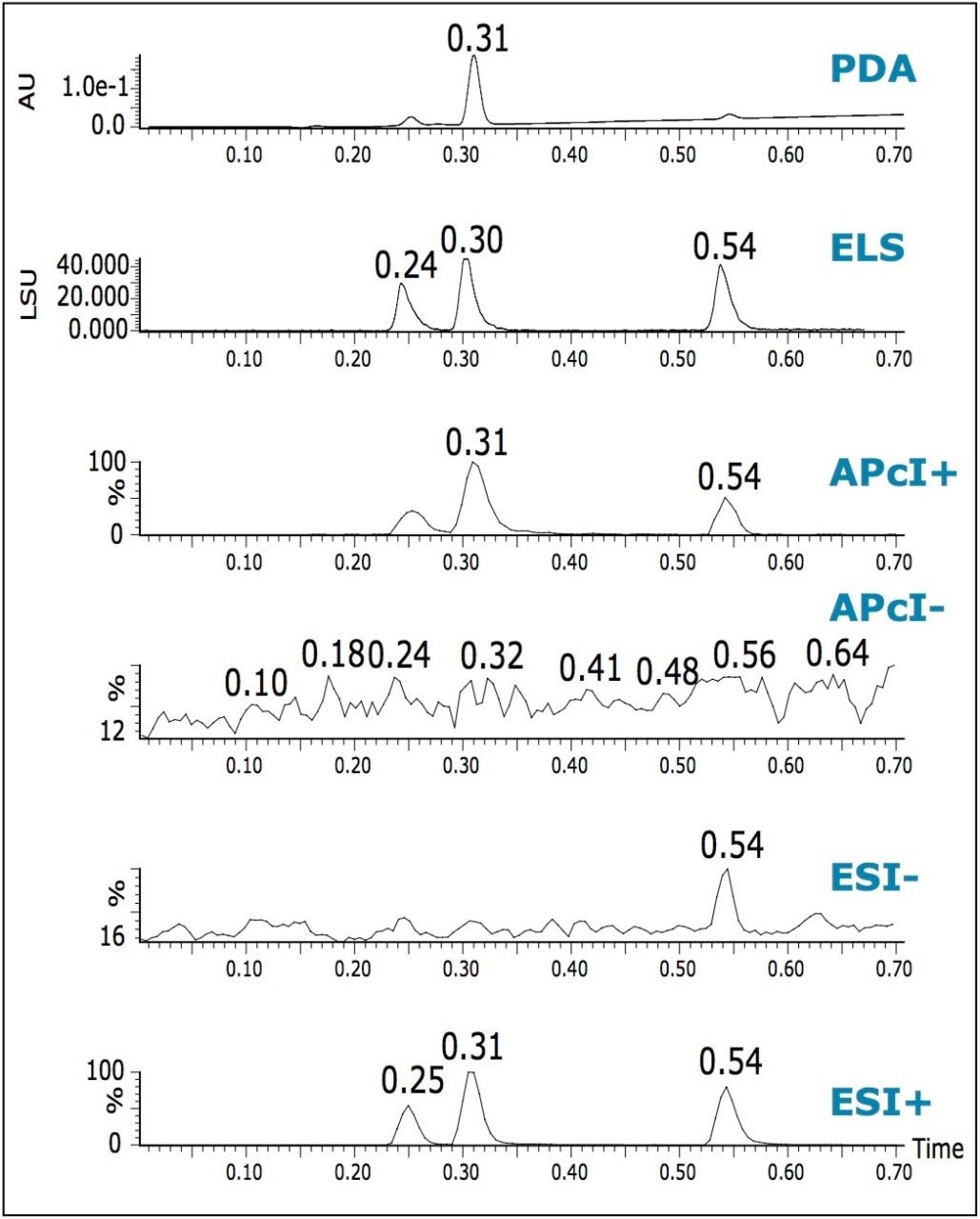
The chromatographic peak widths of the MS and ELS increased by 25 to 30% when compared with the PDA trace. This can be attributed to the use of a low volume micro-tee after the PDA.
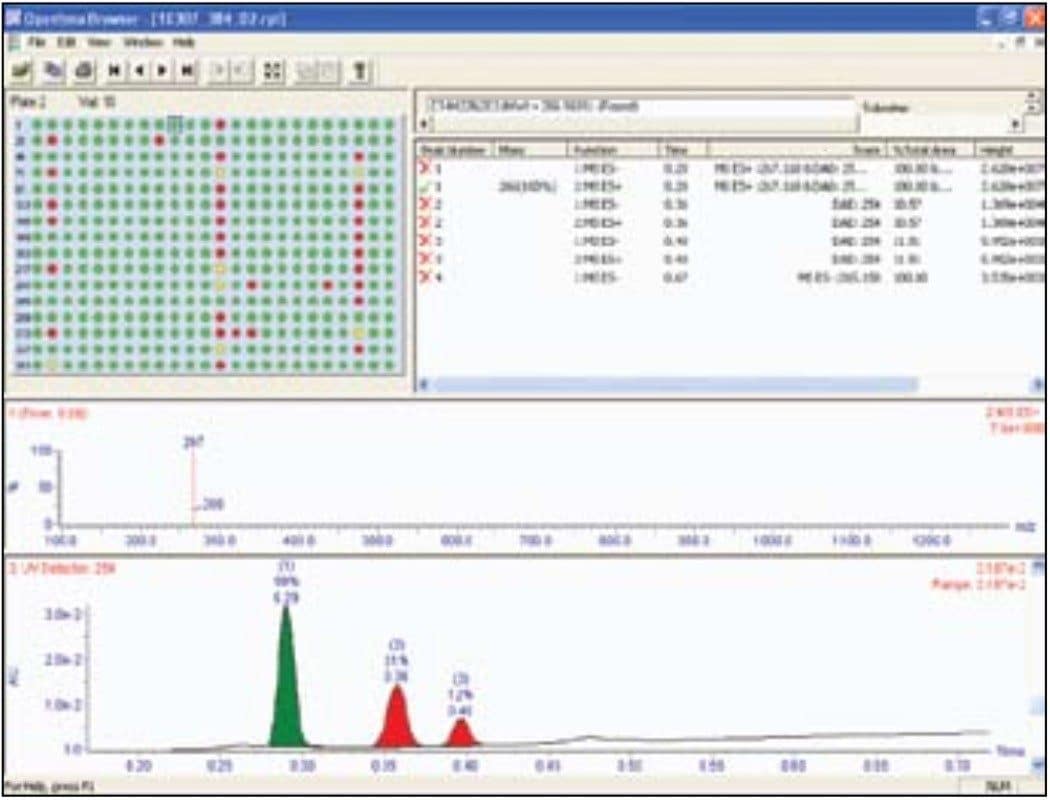
As soon as the analysis is complete, data is automatically processed and a sample report is generated. Reporting in Open Access systems is facilitated by the OpenLynx Application Manager. OpenLynx can report results using printed reports or through the OpenLynx browser. The browser presents a summary of the results as a color coded (found/not found/tentative) map for clear interpretation of the results. Chromatograms, spectra, sample purity, peak height, peak area, retention time, and other information can easily be viewed by the browser. The OpenLynx browser, shown in Figure 6, displays the results for the entire 384-well plate. The report can automatically be emailed, converted to pdf, or printed as desired.3
The OpenLynx OA Toolkit facilitates an even easier administration of an open access system, automating many of the system management tasks carried out by a system administrator. The software also remotely monitors the status (via the Remote Status Monitor module) of one or more acquisition PC s and writes monitoring information to an XML file. The status summary page opens in the browser and contains a list of acquisition PC s, and the number of samples pending in the queue.4 This allows the chemist to select the instrument with the shortest wait time, again increasing productivity.
It is important to verify the identity and purity of a compound before early activity studies. Chemists need to be sure they have synthesized the expected compound. Because large numbers of compounds may be created, it is necessary for this screening to be high throughput. And because only a small amount of material is synthesized, the screening must also consume as little material as possible, while generating a diverse amount of information.
The described system and software combination can autonomously evaluate large numbers of samples with a cycle time of 1 minute and 20 seconds. Data can then be automatically processed and a summary report can be generated. The scan speed capabilities of Waters ACQUITY SQD System make it possible to better characterize narrow chromatographic peaks. This has become a necessity since the advent of sub-2 μm particle technology where chromatographic peaks can be 1 second wide or less.
Signals from auxiliary detectors such as PDA and ELS can be collected simultaneously. Together with the MS data, they provide important information relating to purity and an estimation of the relative quantities of the components present.
Open Access gives the chemist a walk-up system that is flexible for analytical data acquisition. It runs as a complete system, from sample introduction to end results.
The use of the fast-scanning MS along with the throughput of UPLC technology and remote status monitor software allows the chemist to obtain high quality comprehensive data about their compounds in the shortest possible time. This combined with intelligent open access software allows informed decisions to be made faster, thus supporting the needs of the modern drug discovery process.
720002257, June 2007