
This application note demonstrates the profiling of impurities in raw drug substances by applying UPLC.
The evaluation of pharmaceutical raw materials and finished products for impurities and degradation products is an essential part of the drug development and manufacturing testing process. It is a requirement that toxicological information be obtained on any drug-related impurity which is present at a concentration of greater than 0.1% of that of the active pharmaceutical ingredient (API). The impurities produced during the manufacturing process are often related to the solvents employed and raw material source, and so it is essential to compare the impurity profile when scaling from pilot to primary manufacturing, or when moving production from one location to another. It is also necessary to demonstrate the toxicological effects of any drug-related degradation products which are greater than this 0.1% API value.
This impurity profiling is usually performed by HPLC with UV, PDA, or MS detection. As it is essential to detect and measure all of the impurities in the sample, it is necessary to have a high resolution separation process. This usually involves long analysis times resulting in low throughput.
As candidate pharmaceutical compounds become more potent and are dosed at lower and lower levels, there is a requirement for ever more sensitive assays to detect and measure impurities. This low throughput can become the rate-limiting step in product release testing or process evaluation. Any improvement in throughput and sensitivity would be of great benefit to the process of product release and identification of drug-related impurities. Since much of the process of impurity identification involves the coupling of liquid chromatography to sophisticated mass spectrometry equipment, any reduction in analysis time will result in a more efficient use of these significant investments.
UltraPerformance LC (UPLC) exploits the potential of sub-2 μm particles to generate high resolution chromatographic separations. The increased performance generated by utilizing a Waters ACQUITY UPLC BEH C18 1.7 μm Column is demonstrated in Figure 1. Here we can see that when compared to a chemically identical 3.5 μm HPLC particle, the 1.7 μm UPLC material generates superior efficiency. The flat nature of the van Deemter plot produced by these sub-2 μm stationary phases allows these materials to be operated at increased mobile phase linear velocities without any noticeable reduction in chromatographic performance.


The chromatographic separations were performed on a Waters ACQUITY UPLC System, comprising of an ACQUITY UPLC Binary Solvent Manager, an ACQUITY UPLC Sample Manager, Column Manager, and an ACQUITY UPLC Photodiode Array (PDA) Detector. The separations were performed on either an ACQUITY UPLC BEH C18 1.7 μm Column or a Waters XBridge BEH C18 3.5 μm Column.
|
UPLC Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
HPLC Column: |
XBridge BEH C18, 2.1 x 50 mm, 3.5 μm |
|
Flow Rate: |
300 μL/min |
|
Injection Volume: |
1 μL |
|
Column Temp: |
Ambient |
|
Prostaglandin/Pharmaceutical Intermediate Gradient: |
40–100% B, 1–6 min A = 100% Water, B = 100% Acetonitrile |
|
Anti-inflammatory Corticosteroid Gradient: |
20–100% B, 0.2–7 min A = 100% Water, B = 100% Acetonitrile |
|
UV: |
prostaglandins 269–271 nm corticosteroids 239–241 nm |
Corticosteroids are a popular treatment for chronic asthma and rhinitis. These medicines are typical administered as a dry powder or aerosol inhaler. It is a regulatory requirement to be able to detect and quantify all of the impurities in the raw materials used in the manufacturing process, as well as in the final product. This activity is typically carried out by the application of HPLC-UV analysis. For a typical corticosteroid analyzed by HPLC using the USP registered method, an analysis time of 40 minutes was required to generate the expected resolution of the known impurities and degradation products. These long analysis times were needed to generate the high resolution required to resolve/detect all of the impurities/degradation products in the samples.
UPLC was then investigated for the analysis of a common inhaled corticosteroid. Using an ACQUITY UPLC BEH C18 2.1 x 50 mm Column packed with 1.7 μm material with a 40–100% acetonitrile/aqueous gradient over 5 minutes, it was possible to separate all of the known impurities in the sample. The API eluted with a retention time of 5.4 minutes (Figure 2). The UPLC analysis generated a high resolution separation with a total of 12 known impurities resolved and identified by comparison to authentic standards.
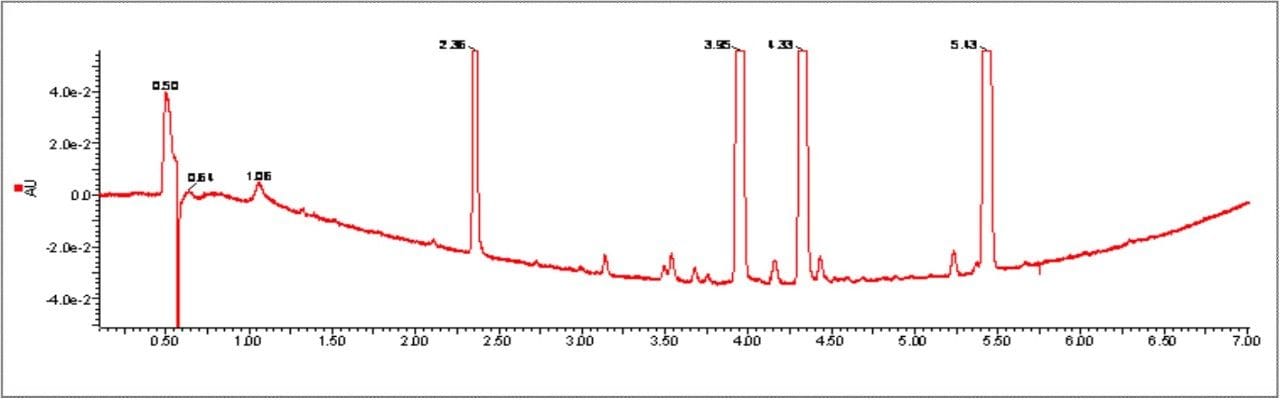
During the development of a pharmaceutical compound, it is necessary to characterize the sample during the chemical synthesis process. The information generated allows for informed decisions to be made during the scale-up from bench synthesis to pilot plant, and then from pilot plant to raw material production. The same analytical procedure was adopted for the analysis of a pharmaceutical intermediate sample.
Again, the original separation was in excess of 40 minutes using a standard 3.5 μm C18 analytical HPLC Column. The application of UPLC to the separation of these analytes resulted in the complete separation of all of the components in less than 5 minutes, as shown in Figure 3.
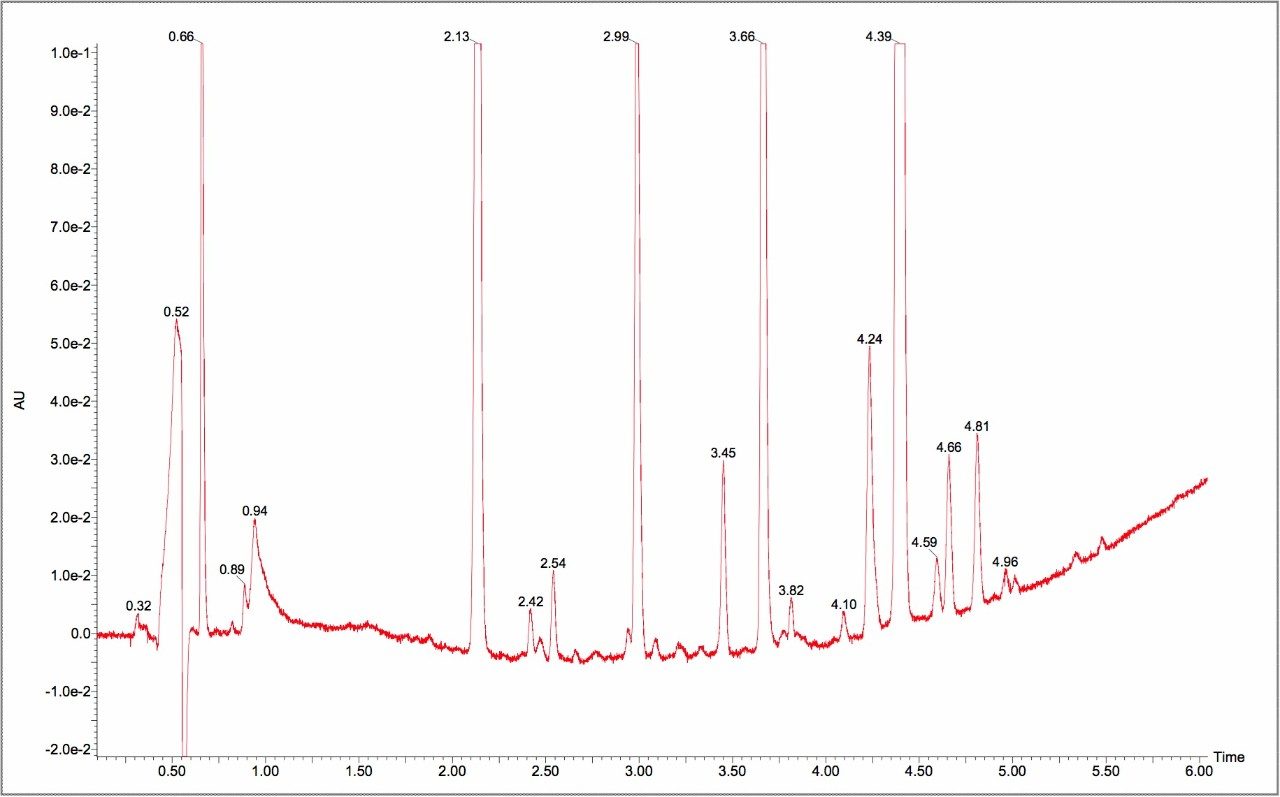
If a new impurity or degradation product is detected in an analytical run, it is necessary to identify and characterize this new component. This often requires the isolation of this component to generate sufficient material to allow proton and carbon NMR experiments to be performed; this is a task often requiring the use of preparative chromatography with UV-or MS-directed fractionation. In order to perform this with UPLC, it is crucial to first transfer the methodology back from UPLC-scale stationary phases to conventional HPLC-scale stationary phases. We have demonstrated a simple approach to this process by using the XBridge stationary phase for the analysis and scale-up of the separation of a crude mixture of prostaglandins. We can see from the data in Figure 4 that the analytical methodology is easily and directly transferable to the chemically identical XBridge 3.5 μm stationary phase, with the selectivity and analysis time maintained from HPLC to UPLC.
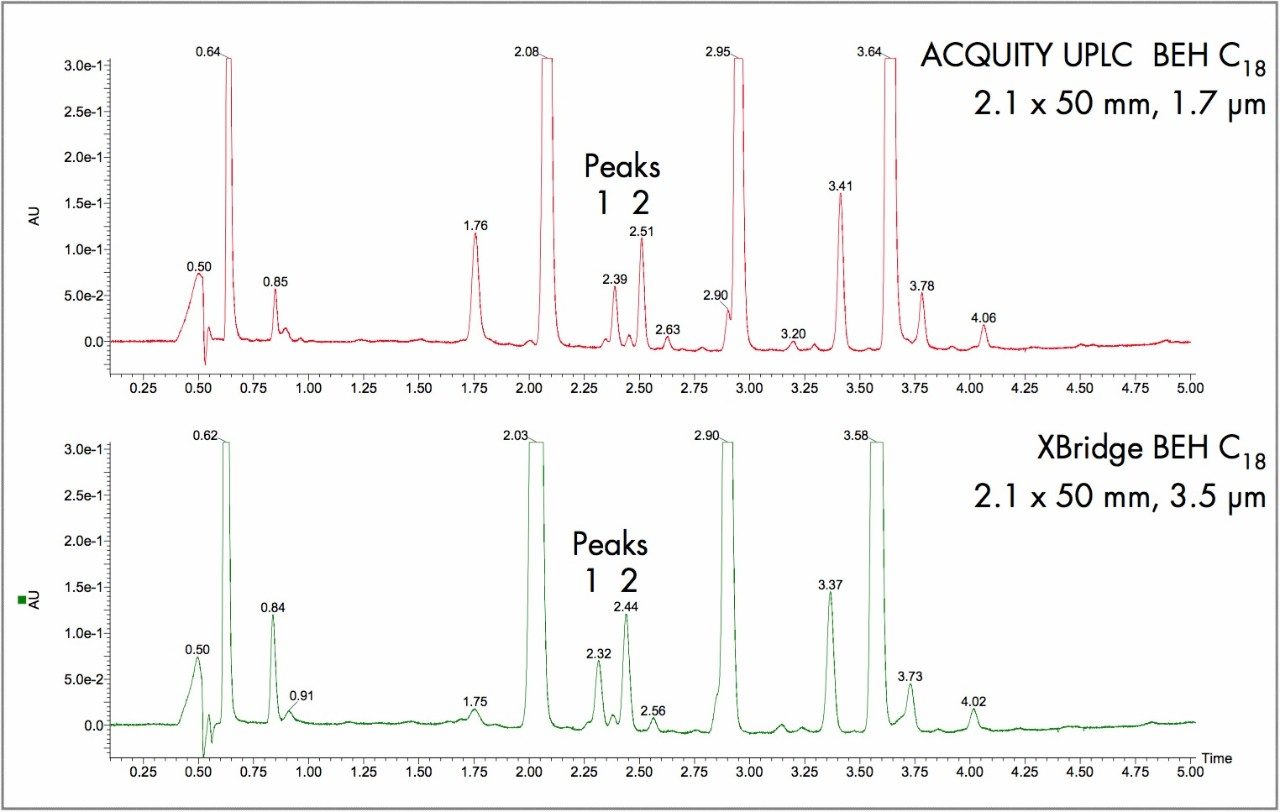
Also, the increased resolution of UPLC is demonstrated by the peaks eluting between 2.25 and 2.75 minutes. In the UPLC separation there are four clearly resolved peaks, whereas with HPLC, the resolution of peaks 1 and 2 is less defined. Additionally, the peak eluting with a retention time of 3.78 minutes has a superior peak shape with UPLC. The elution order of the peaks remains unchanged, as does the retention time. This was achieved by operating the HPLC column with the same mobile phase linear velocity as the UPLC column. In this case, the UPLC system was operated at approximately half the optimal flow rate; the performance of the UPLC separation would improve if the flow rate was doubled and the gradient time reduced by a factor of 2, thus keeping the gradient volume the same. The peak eluting at 0.85 minutes shows a reduced peak height with UPLC compared to HPLC. This is most likely due to extra-column effects. The height of peak eluting at 1.76 minutes increased with UPLC, a result that cannot be explained by the chromatographic effects of reduced particle size and may be a result of compound instability in the autosampler.
The data displayed here demonstrates the high resolution separation generated by UltraPerformance LC. Using the ACQUITY UPLC System, it was possible to produce impurity assays for candidate pharmaceutical raw materials with analysis times of less than 7 minutes. This reduction in analysis time allows approximately 6 more separations to be performed per unit time by UPLC, when compared to that by HPLC.
All of the separations were performed using a simple linear gradient elution profile. It has been shown that UPLC is ideal for the demanding tasks of impurity profiling and degradation product analysis. Additionally, transfer of the UPLC separations to preparative-scale is simplified and streamlined with chemically identical XBridge BEH C18 Columns, for the scale-up to HPLC isolation of new or unknown impurities.
720001465, February 2006