High-Throughput Measurement of Compound Solubility and Physical Form with BMI
Waters Corporation, United States
Published on November 14, 2025
Introduction
Aqueous solubility of small molecule compounds is an essential parameter during the hit-to-lead stage of drug discovery as well as during lead optimization and formulation. Low solubility can impact biological assays in a number of ways, including underestimated potency and toxicity, inaccurate structure-activity relationships (SAR), and difficult to interpret results. Poor bioavailability and underestimated toxicity directly impact downstream formulation and manufacturability of dosage forms – increasing development costs and reducing the chances of a drug candidate’s success.1–3
In this application note, we introduce backgrounded membrane imaging (BMI) on the HORIZON® system as a rapid and sensitive high-throughput method for compound solubility measurement. Particle analysis with BMI is easy to perform, requires as little as 25 of sample, and is unaffected by solvents or other media components. In addition, highly resolved images and shape distribution data provide valuable information on morphology of precipitated solids for more comprehensive solubility characterization.
Results and Discussion
BMI Overcomes Limitations of Other Solubility Screening Methods
Despite its critical importance at early stages, current methods for solubility measurement have limitations that affect reliability and/or time to results (Figure 1). In the more cumbersome multi-step methods, precipitates are removed by filtration or centrifugation, and concentration of compound remaining in solution is measured by various detection technologies such as UV absorbance or mass spectrometry coupled with HPLC. These methods are not only time consuming and inefficient, but can be affected by compound sticking to filters, incomplete removal of low-density aggregates by centrifugation, and interference by solvents and matrix impurities.
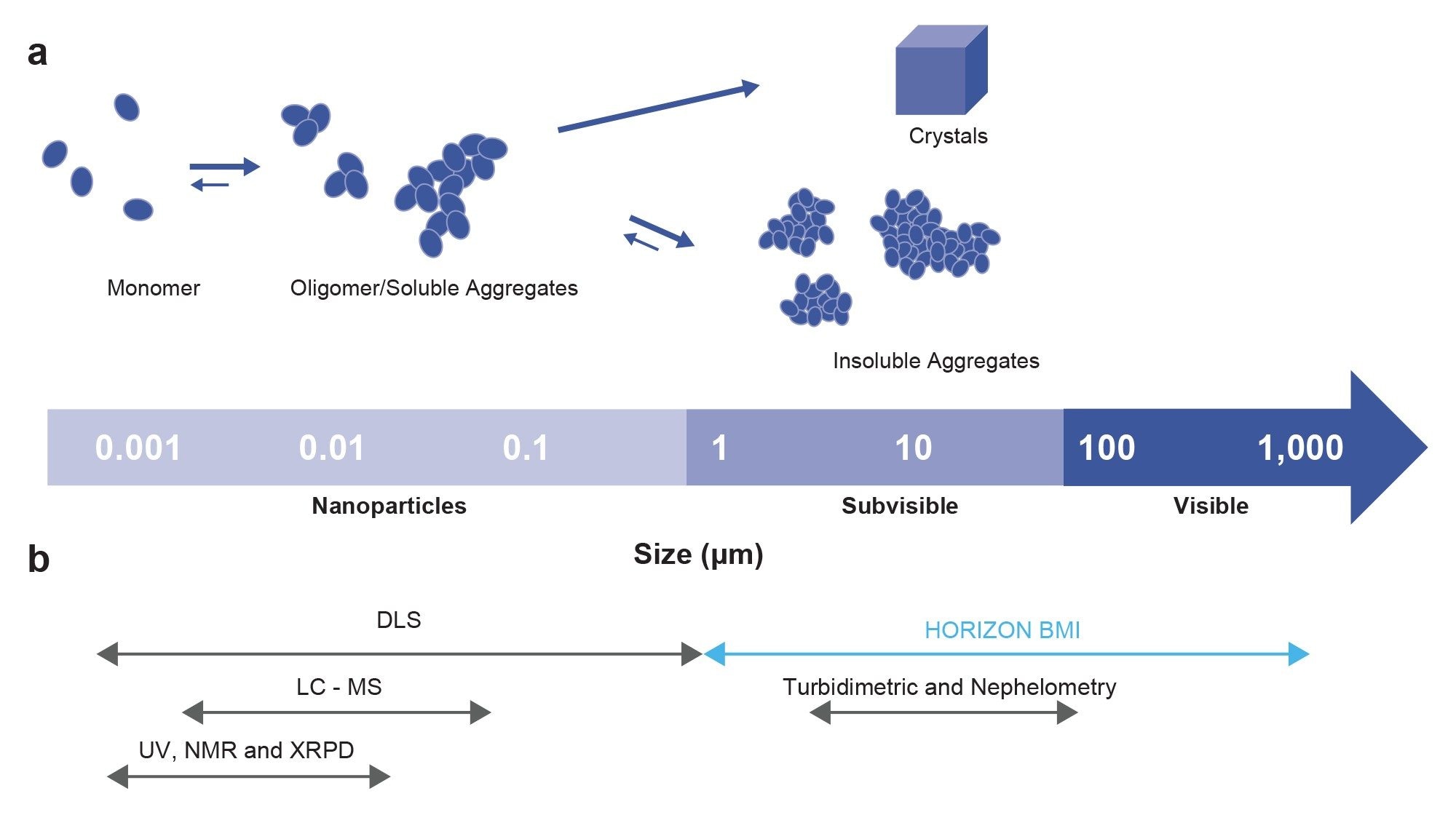
Homogeneous assays such as turbidimetry, nephelometry, and dynamic light scattering (DLS) directly measure compound aggregates in solution. These are simple to perform, but either lack sensitivity or are unreliable for solubility determination. While DLS is the most sensitive of these methods, it cannot provide accurate particle number information when particles have a wide size distribution or larger aggregates are present. BMI is a novel automated microscopy technology that images and analyzes insoluble aggregates captured on a membrane in low-volume, high-throughput format. It offers the ease of use and throughput advantages of homogeneous solution assays but with higher sensitivity and more comprehensive particle data.
Since aggregates are measured on a dry membrane surface and not in solution, presence of solvent, matrix effects, or compound stickiness do not interfere with BMI measurements. Every particle ≥2 µm is counted and analyzed, regardless of particle number or size distribution, so that changes in solubility can be detected even when only a few aggregates are present in the sample.
How BMI Works
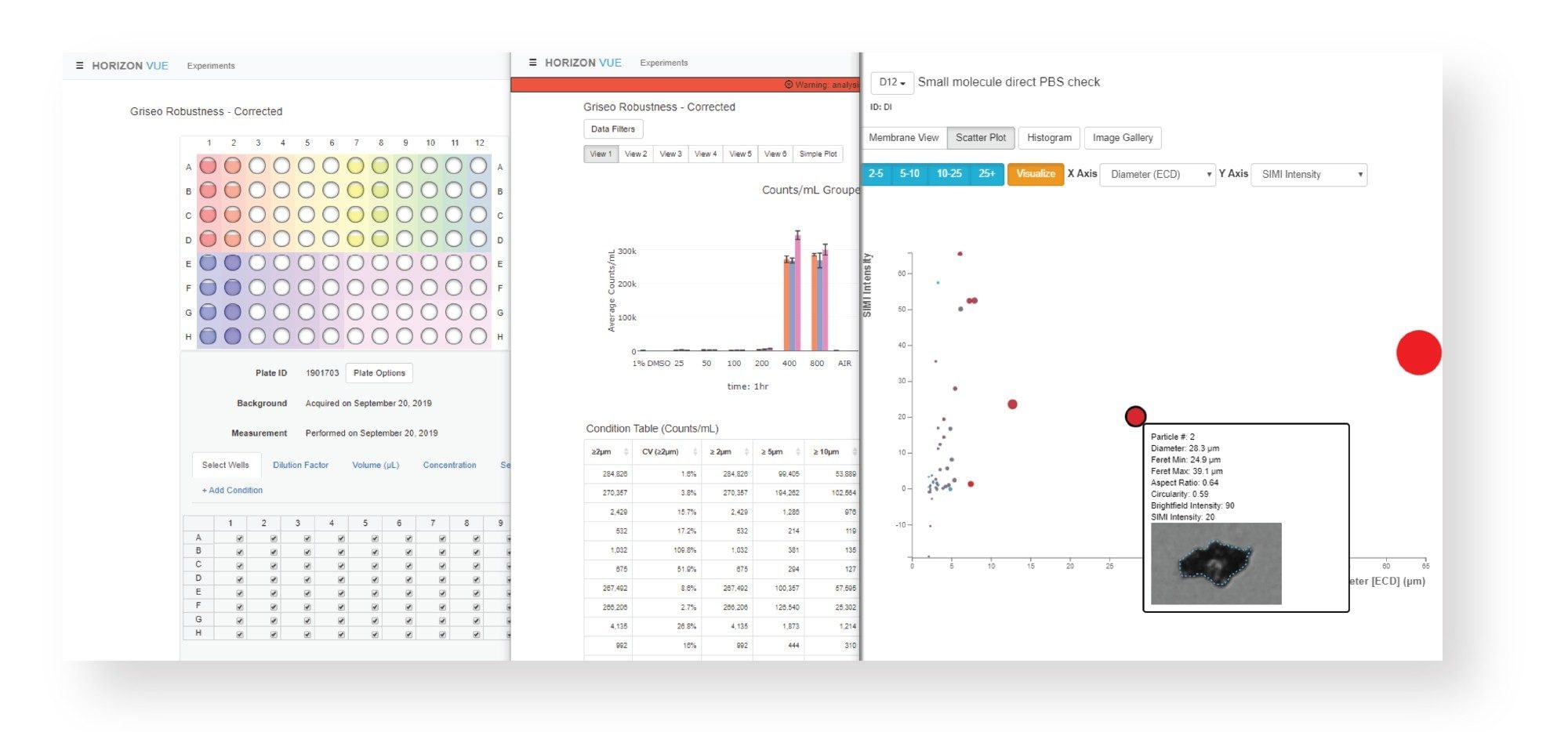
The HORIZON® system system uses sophisticated image-processing techniques coupled with a simple workflow to acquire and analyze particle data. First, the HORIZON® membrane plate wells are measured to generate a background image (Figure 2a). Samples are then pipetted directly onto the membrane wells and filtered through by vacuum, capturing insoluble particles on the surface. The same wells are re-imaged in the HORIZON® instrument. After measuring, the software aligns and processes background and sample images pixel by pixel so that background texture is eliminated, and particles can be viewed in high contrast with minimal interference from membrane surface imperfections (Figure 2b).

Data acquisition on the HORIZON® system is fast, totaling about 1 minute per well for both background and sample image. An entire 96-well membrane plate can be analyzed in less than two hours. Sample processing can be automated on a liquid handling robot. Data analysis tools provide quick and easy access to particle characterization parameters like equivalent circular diameter (ECD), aspect ratio, circularity, etc. Interactive scatter plots and histograms enable visualization of data by multiple characteristics (Figure 3) and calculation of useful statistics such as size and shape distribution and variability.
Subvisible Particle Detection with BMI as a Measure of Solubility
To demonstrate correlation of subvisible particle counts measured on the HORIZON® system to solubility changes, four control compounds with different aqueous solubilities were selected for analysis. A concentration series for each compound was prepared directly from DMSO stocks, then diluted into PBS, pH 7.4 to 1% DMSO final concentration. PBS compound samples were measured on the HORIZON® system, and images analyzed for percent membrane area coverage by particles. Values are graphed in (Figure 4).
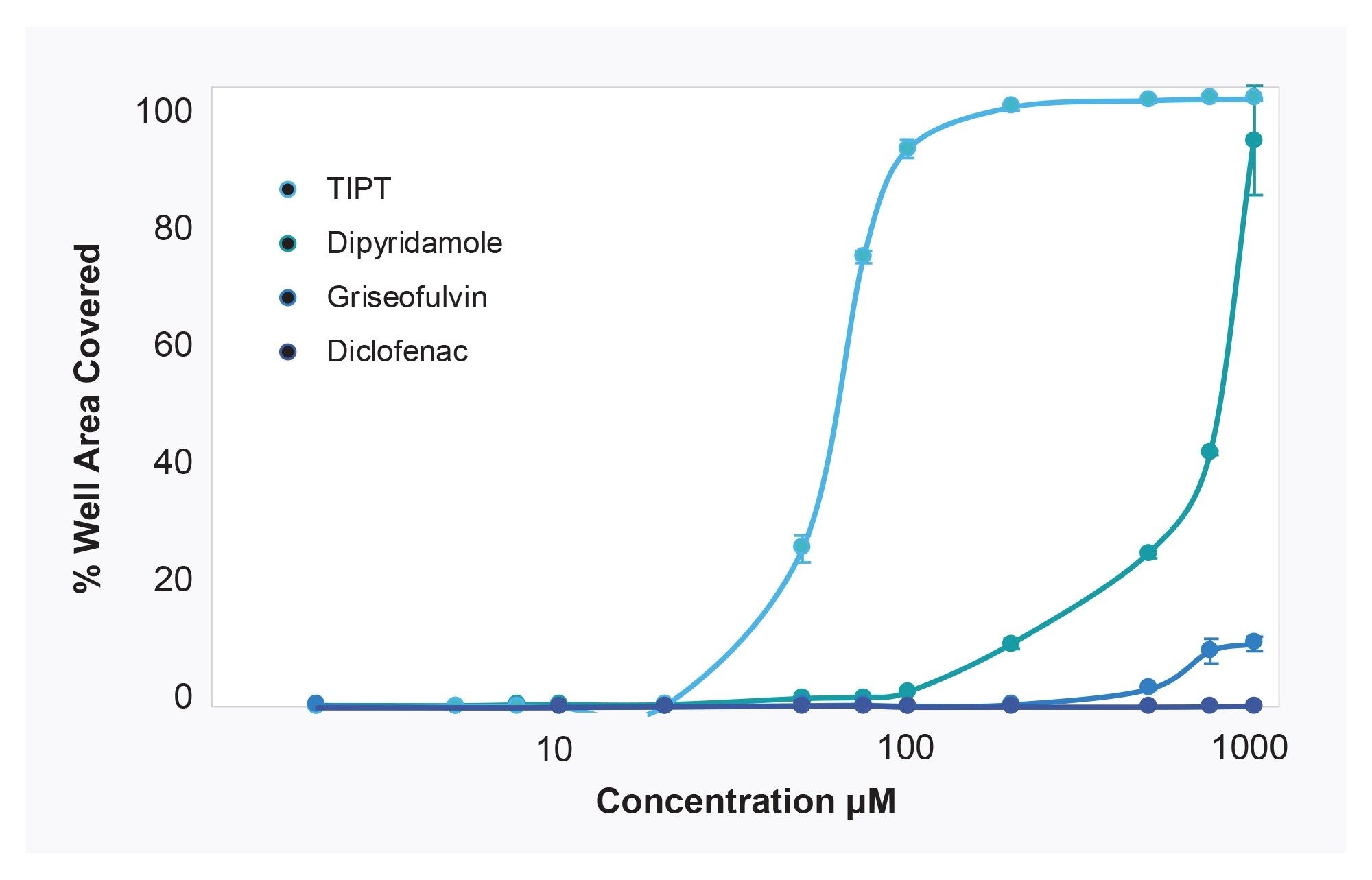
The data illustrate that once insoluble particles are detected in a sample, increasing concentration of compound corresponds to increasing particle coverage on the membrane, until well saturation is reached. Moreover, inflection of the particle coverage curves and rate of increased precipitate formation vary for each compound. TIPT and Dipyridamole aggregates increase at lower concentrations than for comparatively soluble Diclofenac Sodium - where membrane wells were still clear at highest concentration tested. These differing compound profiles support the detection of subvisible particle aggregates as an informative way to look at solubility.
More Sensitive Kinetic Solubility Measurement
Kinetic solubility is primarily measured in early discovery to assess compounds for their ability to be in solution during in vitro assays. At this stage, compounds are usually available as stock solutions in DMSO and measurements are done in high-throughput with small scale volumes. The HORIZON® system is ideal as a discovery screening platform, since up to 96 samples can be measured at once using as little as 25 µL per sample. Yet, the system is easy and flexible enough to be suitable for quick sample precipitation checks or for use in smaller projects. To measure kinetic solubility with BMI on the HORIZON® system, compound dilutions were made directly from DMSO stocks according to the scheme in (Figure 5). After one hour, 50 µL of each sample (3 replicates) was loaded onto membrane wells, which were then imaged and analyzed on the HORIZON®.
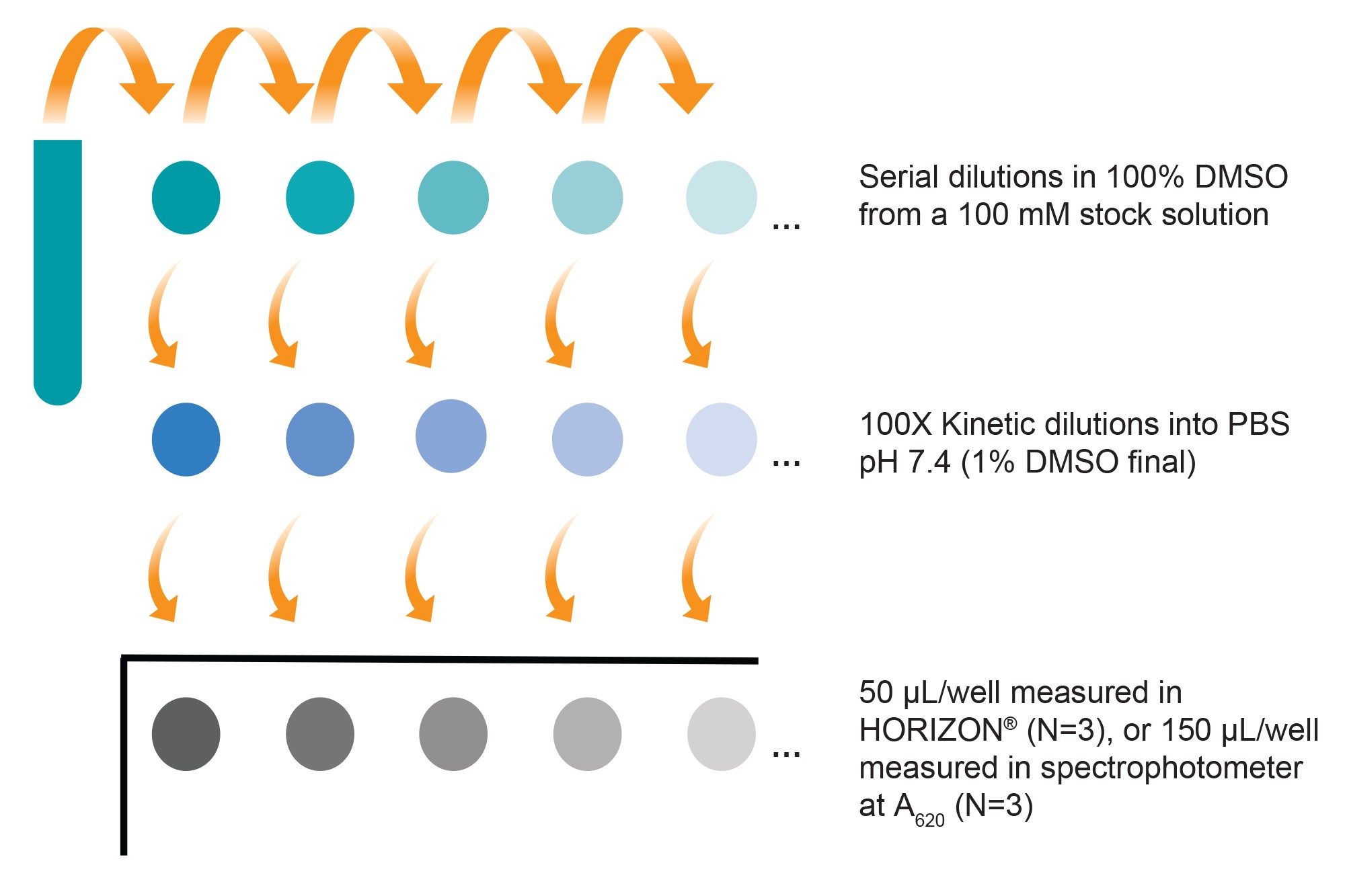
For data analysis, an arbitrary threshold particle coverage value was set at 0.5% of effective well saturation to mark change in solubility (Figure 6). Kinetic solubility range for each compound was reported as the concentration above and below where membrane area coverage by particles exceeded this threshold. The midpoint of this range was assigned as the compound’s estimated solubility.
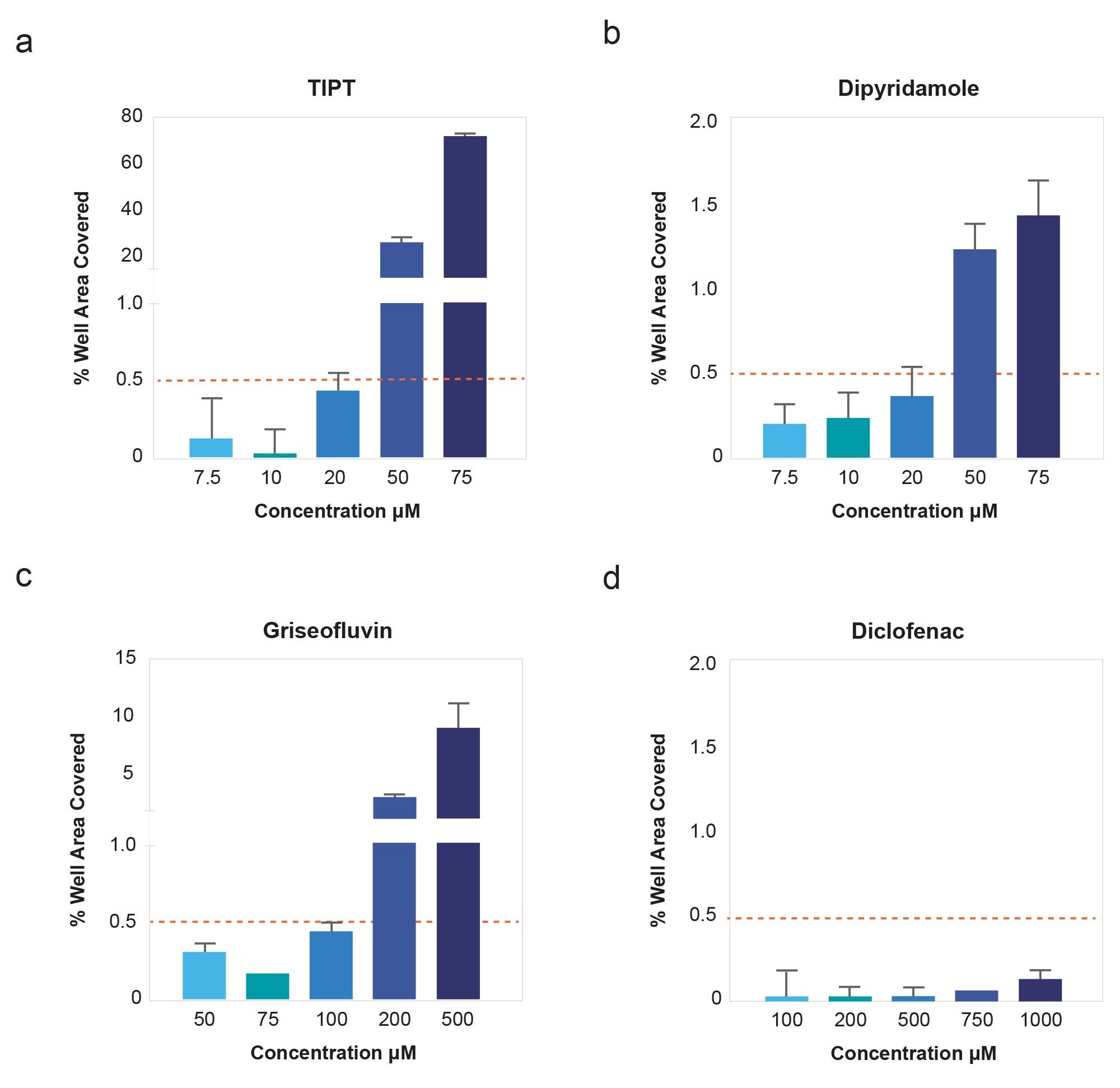
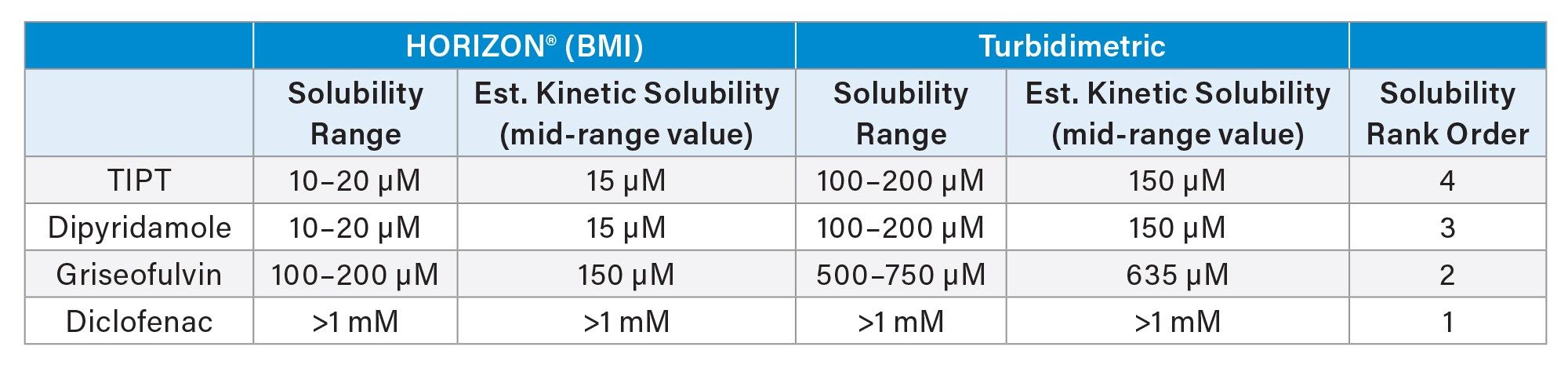
Table 1 lists the kinetic solubilities determined on the HORIZON® system compared to the same samples measured by turbidimetry. Ranking order of the four test compounds for solubility with BMI was identical to ranking by turbidimetry. However, using the HORIZON® particle aggregates were detected at 5–10 times lower compound concentration. BMI’s higher sensitivity enables a more accurate measure of when insoluble precipitates begin to form, making it more reliable for quick and easy identification of problematic compounds.
Particle Images Provide Information on Physical Form
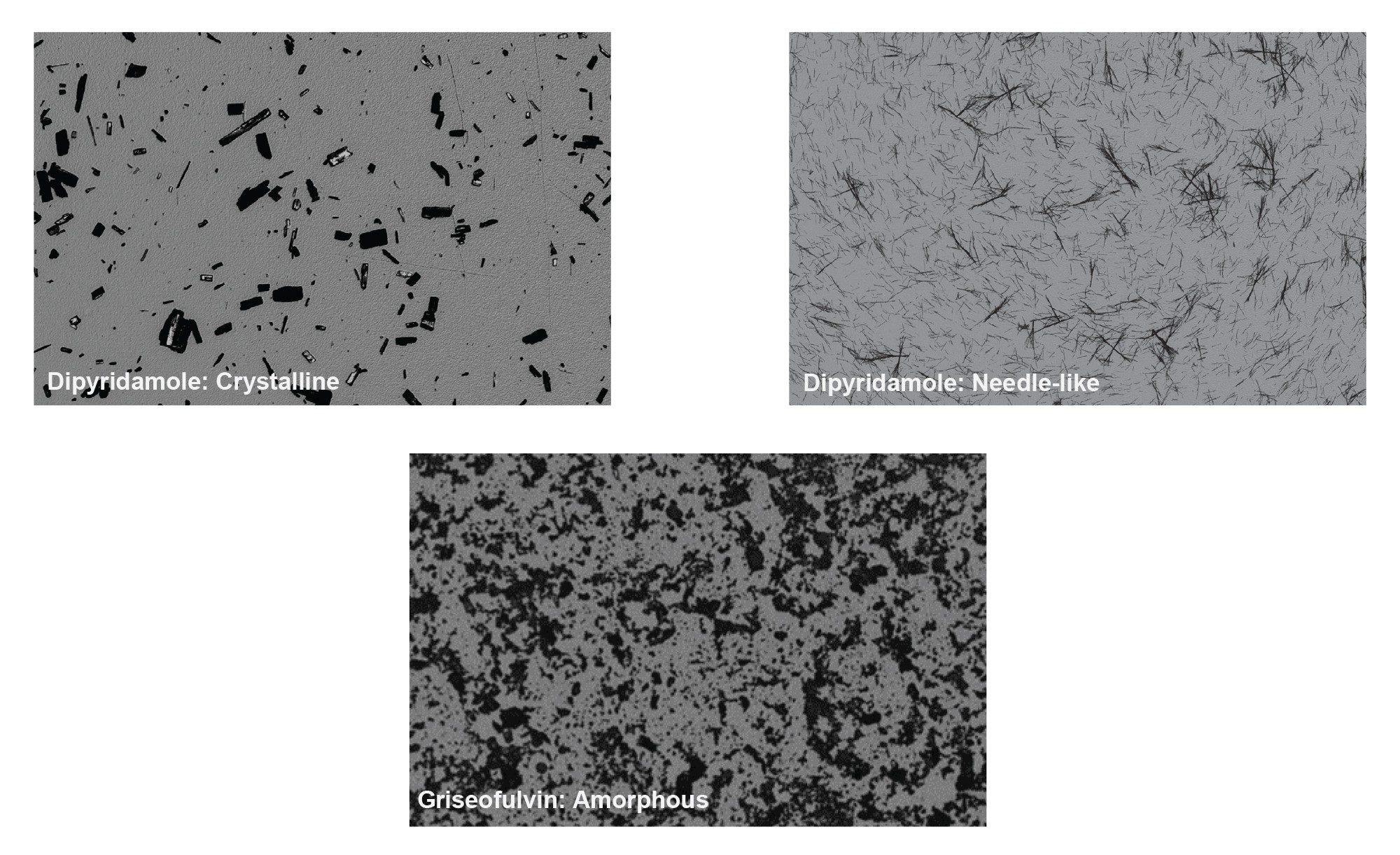
Small scale synthesis of compounds during selection stage can lead to variation of physical form, which can have a dramatic effect on the measured aqueous solubility of compounds. The difference in solubility between amorphous and crystalline forms of the same compound can be up to 1000–fold, and presence of amorphous material can result in a solubility enhancement that can affect in vitro and in vivo behavior.4–5 Evaluating the solid forms of a drug candidate is an important aspect of screening and developability assessment as it can impact downstream optimization. BMI provides high resolution images and digital image analysis of aggregate particles to aid in evaluation of solid-state form. (Figure 7) shows a comparison between images obtained during solubility measurement on the HORIZON® system of compounds in different solid states.
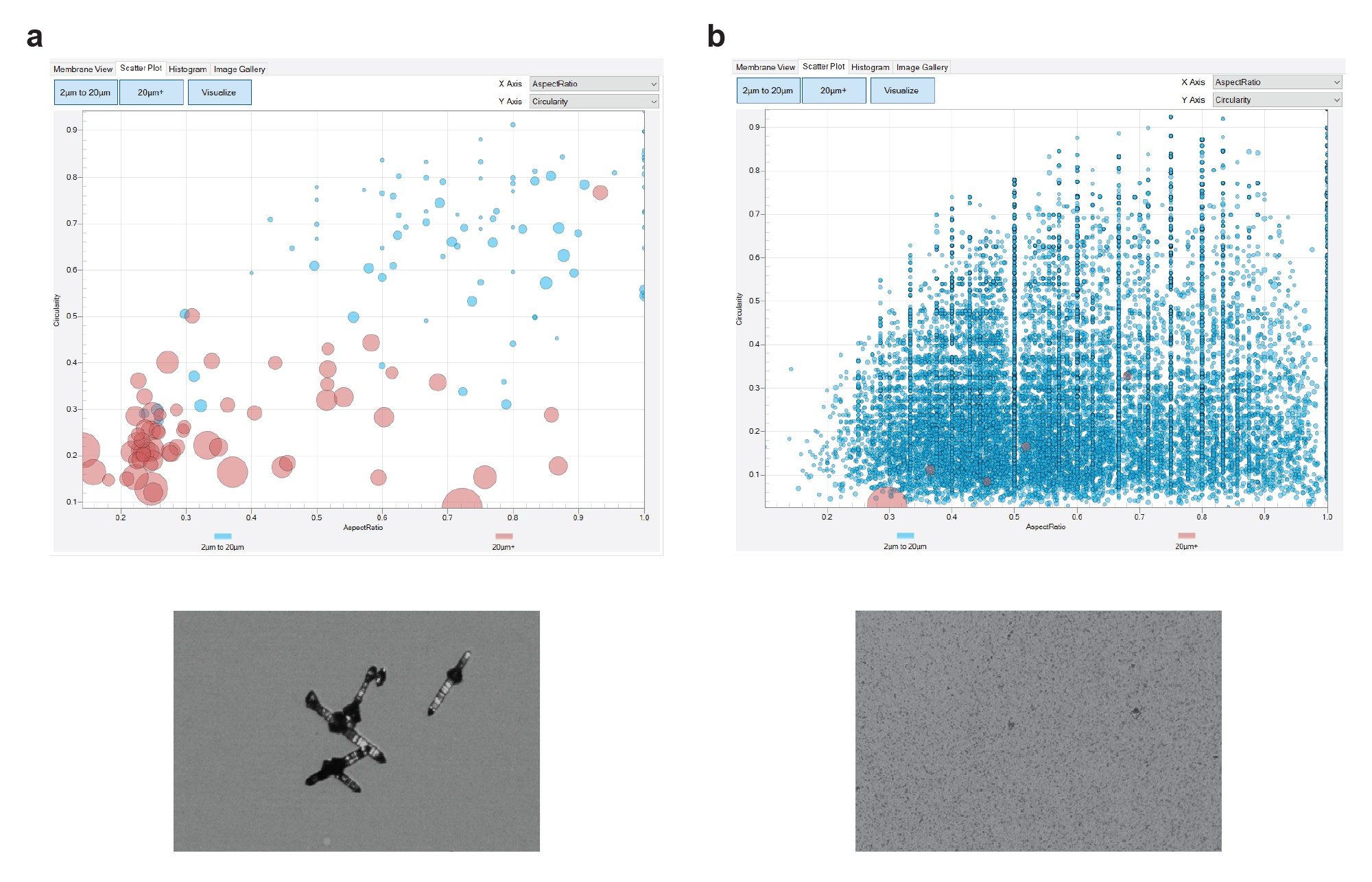
In addition, the HORIZON® software analysis tools provide quantitative data on size and shape distribution of solid particles in the sample (Figure 8). Tools such as the HORIZON® system which elucidate the size and shape of particulates provide significantly more information about the compounds being analyzed than other high-throughput methods. Early access to data on physical form can significantly mitigate risk later in the drug development process.
Conclusion
Here, we have demonstrated how high-throughput membrane imaging with BMI on the HORIZON® system offers reliable, informed solubility assessment of small molecule compounds. In addition, imaging provides valuable information on solid state morphology for better interpretation of solubility results. Whether used as a primary solubility screening method, an orthogonal technique, or as a check to eliminate problematic candidates prior to in-depth characterization, implementing BMI technology can accelerate drug discovery and early ADME workflows - providing more complete information with less time and less material than traditional methods.
References
- Wan H. What ADME tests should be conducted for preclinical studies? ADMET & DMPK. 2013 Jul; 1(3): 19–28.
- Andrade EL, Bento AF, Cavalli J, et al. Non-clinical studies in the process of new drug development - Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies. Braz J Med Biol Res. 2016 Dec; 49(12).
- Strovel J, Sittampalam S, Coussens NP, Hughes M, Inglese J, Kurtz A, et al. Early drug discovery and development guidelines: For academic researchers, collaborators, and start-up companies. In Assay Guidance Manual [Internet]. Eli Lilly & Company and the National Center for Advancing Translational Sciences. 2004.
- Tong WQ. Practical Aspects of Solubility Determination in Pharmaceutical Preformulation. In Solvent systems and their selection in pharmaceutics and biopharmaceutics. Springer New York. 2007 Aug; vol. 6: p.137-149.
- He Y, & Ho Chris, Yang D, Chen J, Orton E. Measurement and Accurate Interpretation of the Solubility of Pharmaceutical Salts. Journal of Pharmaceutical Sciences. 2017; 106.
Featured Products
720009139, November 2025