ACQUITY™ RDa™ Detector with RemoteAnalyzer® Software for Open-access Mass Confirmation of Synthetic Targets
Christopher Henrya, Michael Jonesa, Scott J Campbellb
a Waters Corporation, United States
b SpectralWorks, United Kingdom
Published on November 14, 2025
Abstract
In this application note, a rapid, simple sample submission for the acquisition of accurate mass measurements using the ACQUITY RDa Detector is demonstrated. With the fully integrated walk-up RemoteAnalyzer software, targeted screening workflows are accessible to both expert and non-expert mass spectrometrists to accelerate productivity during the drug development process.
Benefits
- Routine access to targeted accurate mass measurements for confident drug target identification
- Single, simple, and intuitive sample submission interface
- Browser-based acquisition enabling easy, instantaneous data sharing across geographies/sites
- Completed results can be emailed or exported to an ELN (electronic notebook)
- Compatible with all waters_connect™ and MassLynx™ instruments
Introduction
For medicinal chemists, mass spectrometry (MS) is increasingly being relied upon for confirmation of the successful synthetic route design of drugs through identification of synthetic targets and intermediates.1
The need for continuous productivity increases in drug development has also led to an increased reliance on open-access software.2 This is both to facilitate a simplified sample submission process and the receipt of condensed, easily digestible results for rapid decision making.
Traditionally, these workflows have included liquid chromatography (LC) coupled to ultraviolet (UV) detection and/or nominal mass quadrupole mass spectrometers. Nominal mass data, while useful, does not eliminate the potential of mis-assignment of the target compound. If employing nominal mass identification workflows, orthogonal detection techniques like nuclear magnetic resonance (NMR) are required for confirmation, which increases the time to answer. This is especially important in cases where additional synthetic product is needed to characterize low level impurities and side products. Increased confidence through MS-based compound characterization alone is possible through the accurate mass measurements provided by High Resolution Mass Spectrometry (HRMS). However, many HRMS systems can require high levels of expertise to operate.
The ACQUITY RDa Detector is an easy-to-use HRMS detector with automatic push button setup, which makes accurate mass data accessible for identification workflows without the need for HRMS expertise. Combined with the open-access software RemoteAnalyzer (SpectralWorks Ltd., Runcorn, UK), chemist end-users now have a simple, quick sample submission interface to accelerate the drug development process. Using targeted analysis enables chemists to confidently confirm a chemical hit without the need for additional testing.
To demonstrate the benefits of this platform, a sample of the anti-diabetic drug glipizide was degraded under acidic conditions to represent a synthesized drug with impurities. Using the “Target Confirmation” workflow within the RemoteAnalyzer Software, accurate mass screening for the glipizide parent drug and two known impurities (Impurities II/III)3,4 (Table 1) were performed.
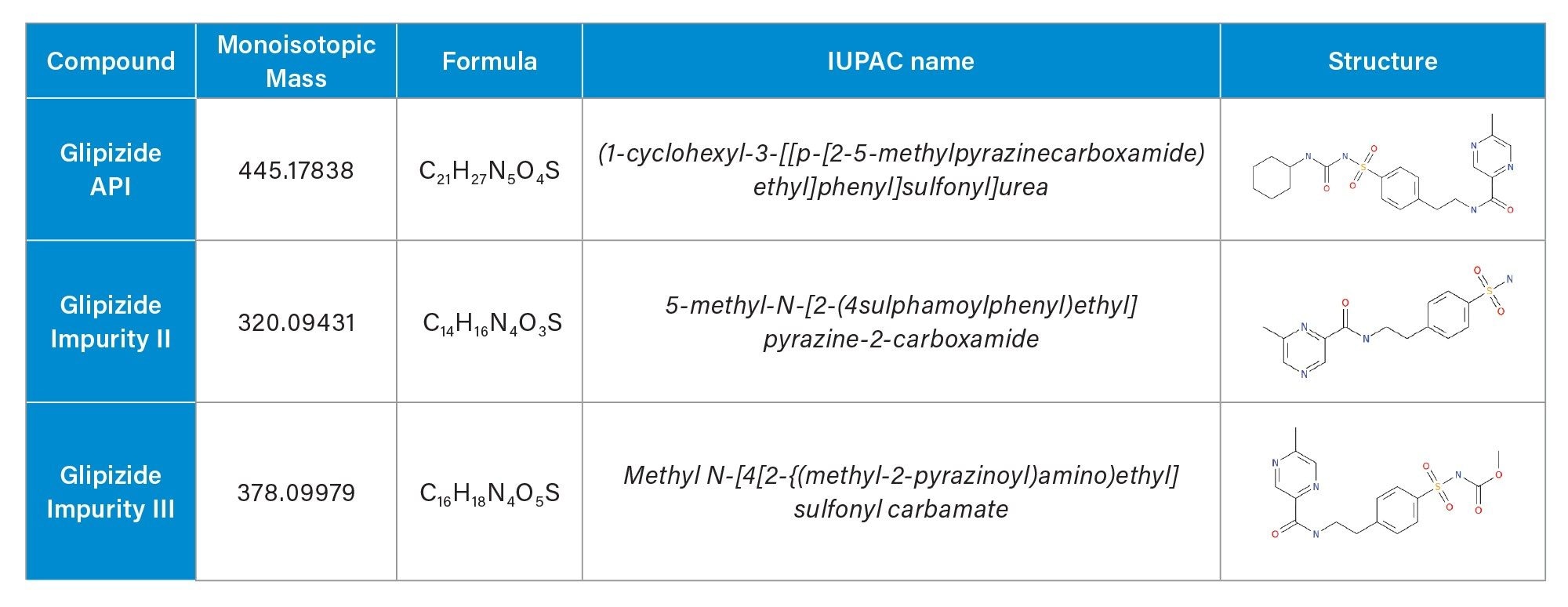
Experimental
Sample Description
Samples of glipizide were prepared and analyzed as per the application note 720007510.4
Data Management
|
MS software: |
waters_connect 4.0.0 |
|
Informatics: |
RemoteAnalyzer® Software v4.61.4678.0, (SpectralWorks Ltd. Runcorn, UK) |
Results and Discussion
When generating data on the waters_connect Platform using RemoteAnalyzer as the user interface software, samples can be submitted singly, as a batch (manually or through a csv. file) or as a 96-well plate using a csv. file. For this experiment, a single sample was submitted manually.
The chemically stressed glipizide sample was submitted using the ‘Target Confirmation’ workflow. The relevant project/experiment was selected from a dropdown menu with the latter containing detection thresholds, saturation threshold, and ppm error limits etc.
In the ‘Formulas’ section, the empirical formula for glipizide and the impurities II and III were entered and labeled with the desired adduct chosen (Figure 1).
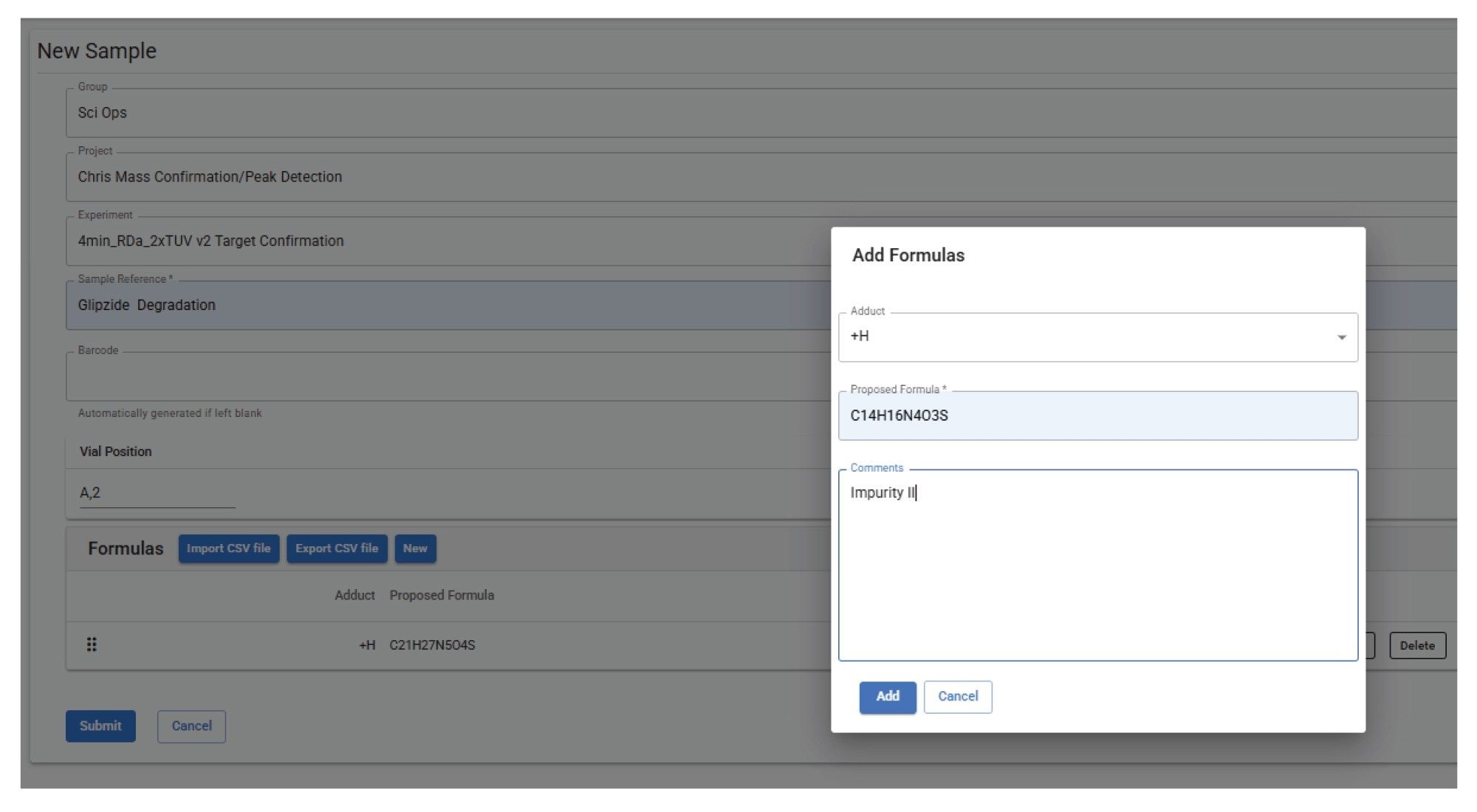
On submission, an email was received to inform that the sample had been submitted. On completion of the sample, a PDF containing ultraviolet (UV)-aligned mass confirmation trace for all three requested empirical formula and mass spectra associated with each detected peak; glipizide API, ppm error 2.57 (Figure 2); impurity II, ppm error 0.66 (Figure 3); and impurity III, ppm error 1.14 (Figure 4).
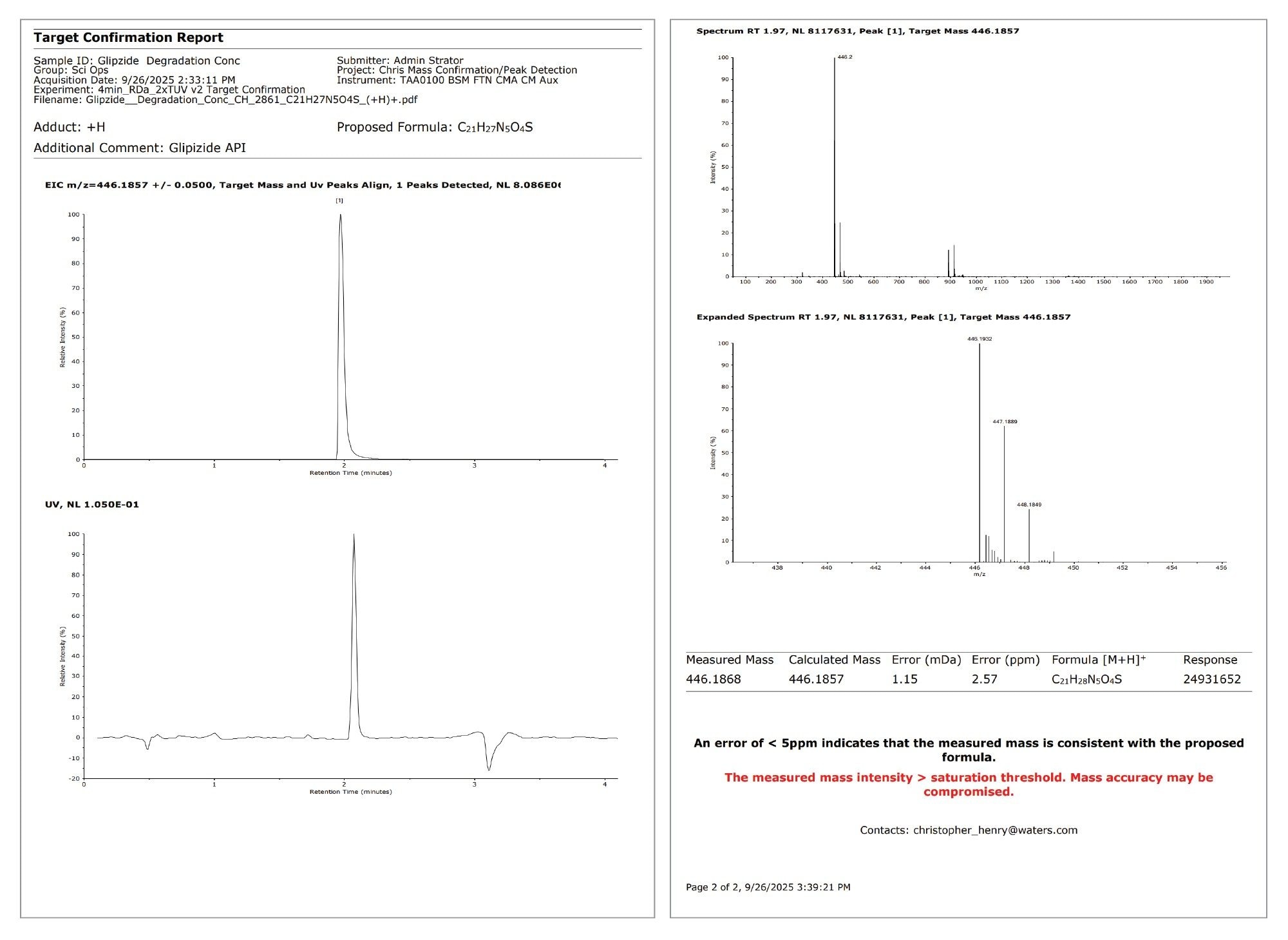
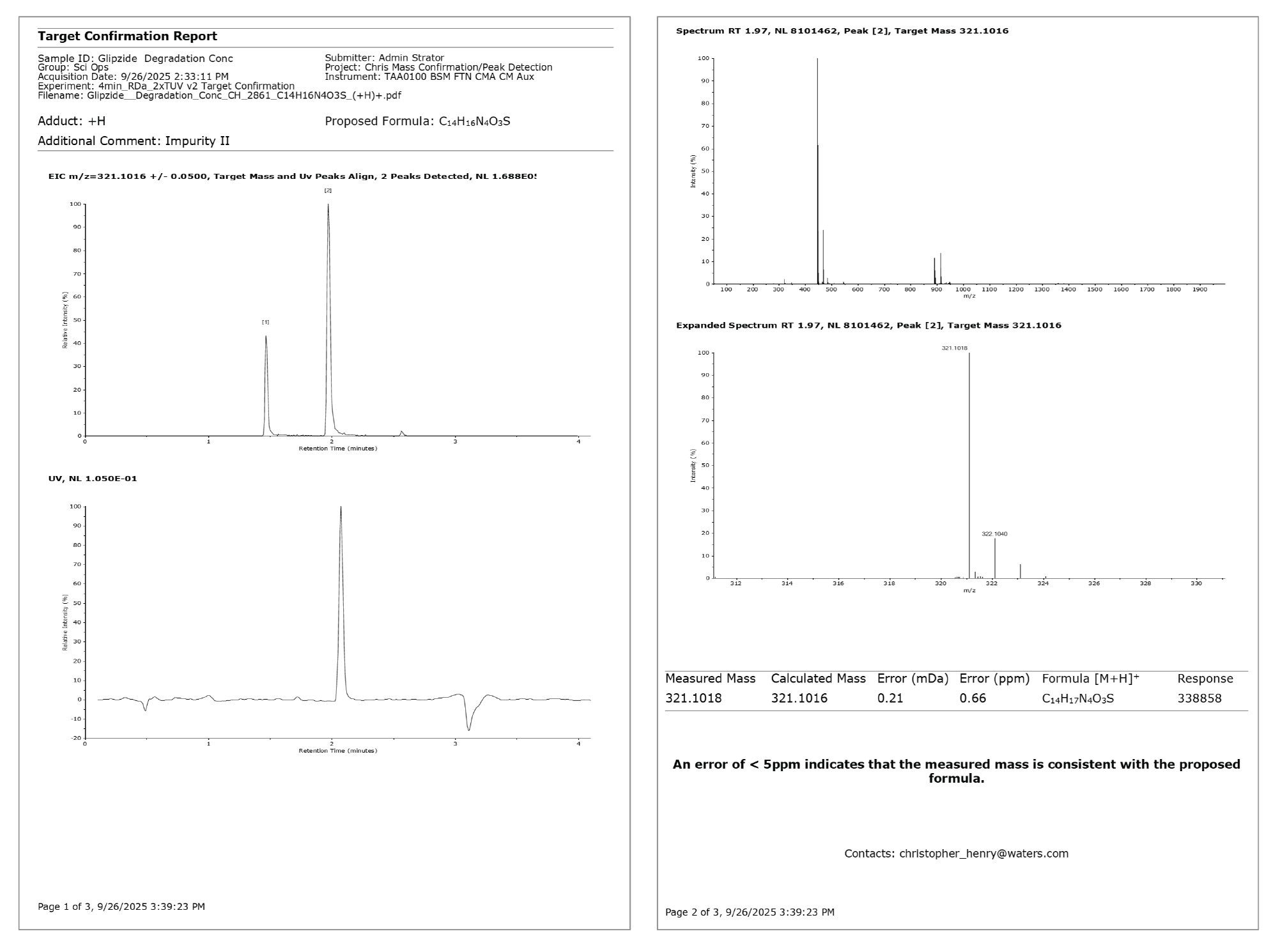
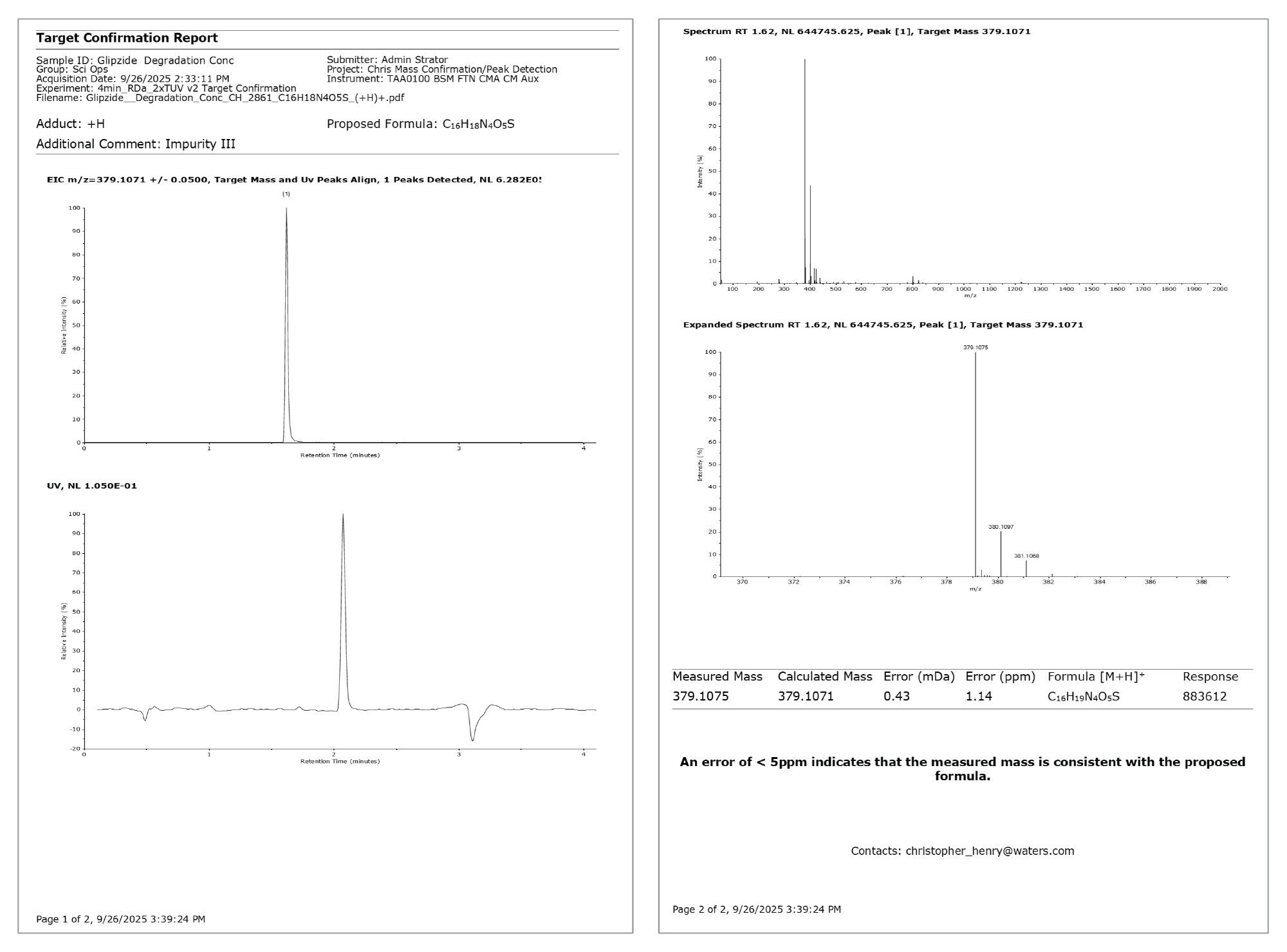
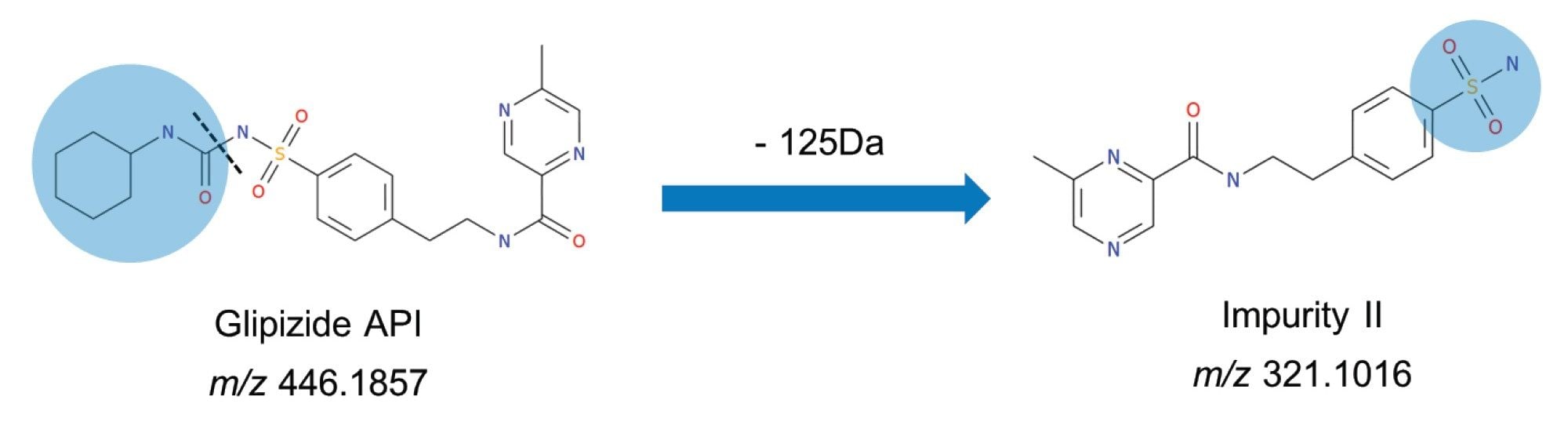
The report for the glipizide API highlighted that the intensity of the detected peak exceeded the saturation threshold stipulated in the processing method. The EIC for impurity II contained two peaks, with the larger peak appearing at the same retention time as the API. This is due to in-source fragmentation cleaving the API at the same site as the hydrolytic cleavage under acidic degradation at high temperatures, i.e., sulfonylurea functional group forming a sulfamoyl group (Figure 5).
Conclusion
In medicinal chemistry, mass spectrometry is a useful tool for delivering increased confidence in the confirmation of a successful chemical synthesis. Walk-up analysis is increasingly being relied upon to expedite sample submission as it facilitates minimal interaction with the instrument, and delivers results in an easily digestible format.
With the ACQUITY RDa Detector controlled by RemoteAnalyzer software, accurate mass measurements are possible via a simple, quick sample submission interface. Empirical formula were submitted for glipizide API and two known impurities; sub-5 ppm accurate mass confirmation results were calculated for all compounds screened. PDF results were emailed with minimal interaction with the LCMS.
With sample submission and processing controlled by the browser-based RemoteAnalyzer Software, chemists can retrieve and review data remotely from anywhere without needing to return to the PC controlling the instrument.
References
- Deng G, Sanyal G. Applications of mass spectrometry in early stages of target based drug discovery. Journal of Pharmaceutical and Biomedical Analysis. Volume 40, Issue 3, 24 February 2006, Pages 528–538,https://doi.org/10.1016/j.jpba.2005.08.038.
- Fontana A, Iturrino L, Corens D, et al. Automated open-access liquid chromatography high resolution mass spectrometry to support drug discovery projects. J Pharmaceut Biomed Anal. 2020;178:112908.
- Bansal G, Singh M, Jindal KC and Singh S. LC and LC–MS Study on Establishment of Degradation Pathway of Glipizide Under Forced Decomposition Conditions. Journal of Chromatographic Science, Vol. 46, July 2008.
- Henry C, Rainville P. Targeted and Non-Targeted Identification and Characterization of the Forced Degradation Products of Glipizide Using the ACQUITY RDa Detector and UNIFI Software Workflows. 720007510, February 2022.
720009118, November 2025