Mass Confirmation and Purity Analysis of PCR Primer and Probe Reagents Using High Sensitivity Ion-pairing LC-MS
For in vitro diagnostic use. Not available in all countries.
Abstract
The SARS-CoV-2 pandemic has brought with it regular testing for infection using RT-qPCR kits. Efforts for this type of disease monitoring benefit from the use of certified, high quality qPCR reagents. In this application note, we describe the use of optimized IP-RPLC and a compliance-ready benchtop Tof instrument for the quality assessment of PCR test reagents used in the SARS-CoV-2 CDC assay. With this sensitive approach, we were able to glean significant amounts of purity information from 10 pmol quantities of nucleocapsid primers N1 and N2 (in both forward and reverse sequences) and the N1 and N2 probes. All PCR reagents yielded sharp, symmetrical peaks. Negative ion mass spectra were acquired and multiply charged species (up to [M-10H]10-) were readily observed, allowing us to unambiguously determine the molecular weights of the RT-qPCR kit reagents. The purity of reagents and unpurified crude materials were evaluated using a combination of both UV and MS detection. As such, an interesting example of a 42.9 mass shifted impurity of the N2 probe was explored. In all, this methodology for combining high resolution, high sensitivity LC-UV-MS was shown to provide a deep level of information that would be of benefit to the certification of RT-qPCR reagents.
Benefits
- Diisopropylethylamine ion pairing applied to PCR reagent analysis for high chromatographic resolution and sensitive UV-MS BioAccord System for online MS detection and accurate mass peak identification
- ACQUITY Premier Chromatography System applied to PCR reagent characterization
Introduction
According to the World Health Organization, the current SARS-CoV-2 pandemic has already caused over four million deaths (September 2021).1 Over five billion vaccine doses have been administrated around the world,1 but unfortunately the availability and rate of acceptance of the available vaccines varies greatly. Transmission of a virus between people over an extended period of time, and subsequently the accumulation of mutations, increases the chance of a significant change in the pathogen’s genome and of a potential increase in its transmissibility or infectivity.2 To date, an essential element of mitigation efforts against the spread of SARS-CoV-2 has been regular testing by means of reverse transcriptase quantitative polymerase chain reactions (RT-qPCR).3,4 The US Food and Drug Administration (FDA) has granted an emergency use authorization to Center for Disease Control and Prevention (CDC) 2019-nCoV RT-qPCR kits. This test is based on the detection of two different locations in the nucleocapsid RNA gene (N1 and N2), and the limit of detection of the assay is approximately 65 viral copies/µL.4 Importantly, the sensitivity of the assay can be dependent on nucleotide substitutions in the primer/probe binding region,3 RNA extraction kit reagents quality,4 and oligonucleotide synthesis errors.5 Analytical approaches to investigate and confirm the quality of PCR test reagents are therefore important. Here, we use ion-pairing reversed-phase chromatography coupled to time-of-flight (Tof) mass spectrometry for high purity assessment of RT-qPCR primer/probe kits.
Experimental
Sample Information
Chemically synthesized oligonucleotides were obtained from vendors as prepared for immediate use in SARS-CoV-2 assays as well as desalt only crude materials. These samples were prepared at 10 pmol/μL with nuclease-free 10 mM Tris, 0.1 mM EDTA, pH 7.5 buffer, and transferred to polypropylene 300 μL autosampler vials (p/n: 186002639).
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class System |
|
Detector: |
ACQUITY UPLC TUV Detector |
|
Wavelength: |
260 nm |
|
Column: |
ACQUITY Premier BEH C18, 2.1 x 50 mm, 130 Å, 1.7 µm |
|
Column temp.: |
60 °C |
|
Sample temp.: |
4 °C |
|
Injection: |
1 μL (10 pmol) |
|
Flow rate: |
0.3 mL/min |
|
Mobile phase A: |
0.1% N,N-diisopropylethylamine (DIPEA) as the IP reagent and 1% 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) in deionized water |
|
Mobile phase B: |
0.0375% DIPEA and 0.75% HFIP in 65:35 acetonitrile/water |
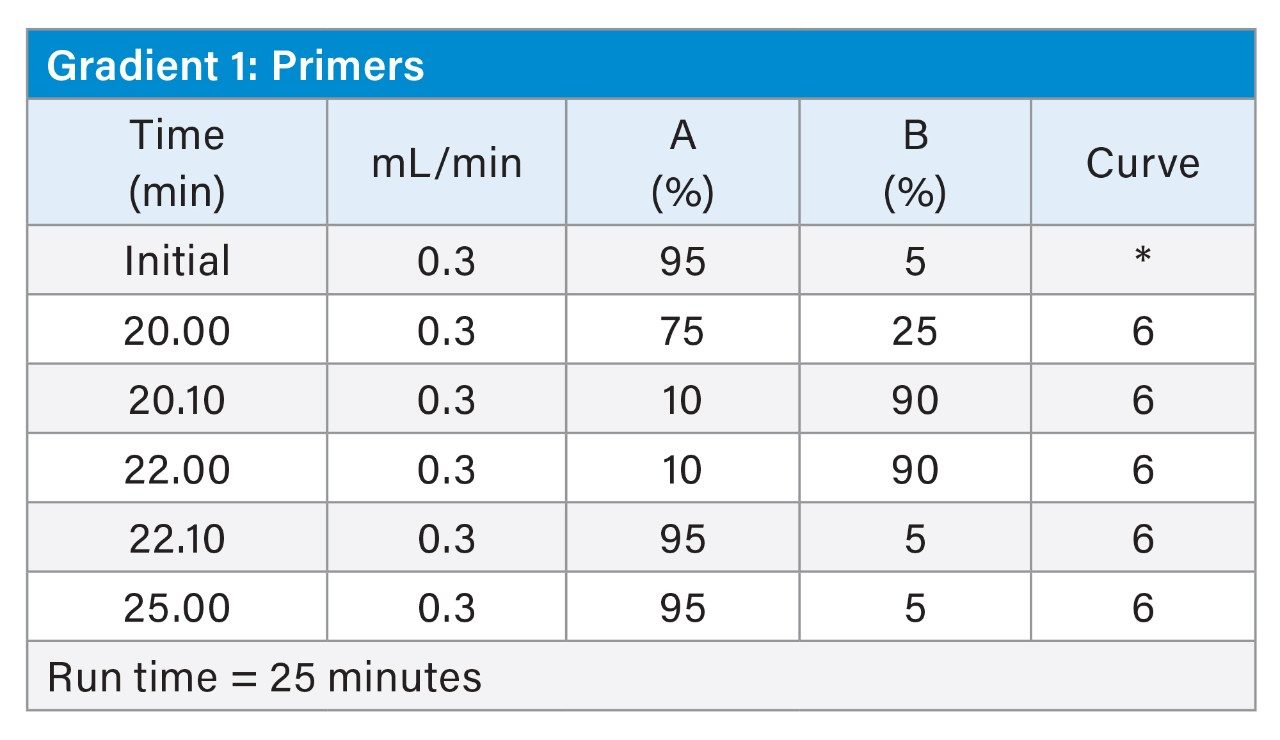
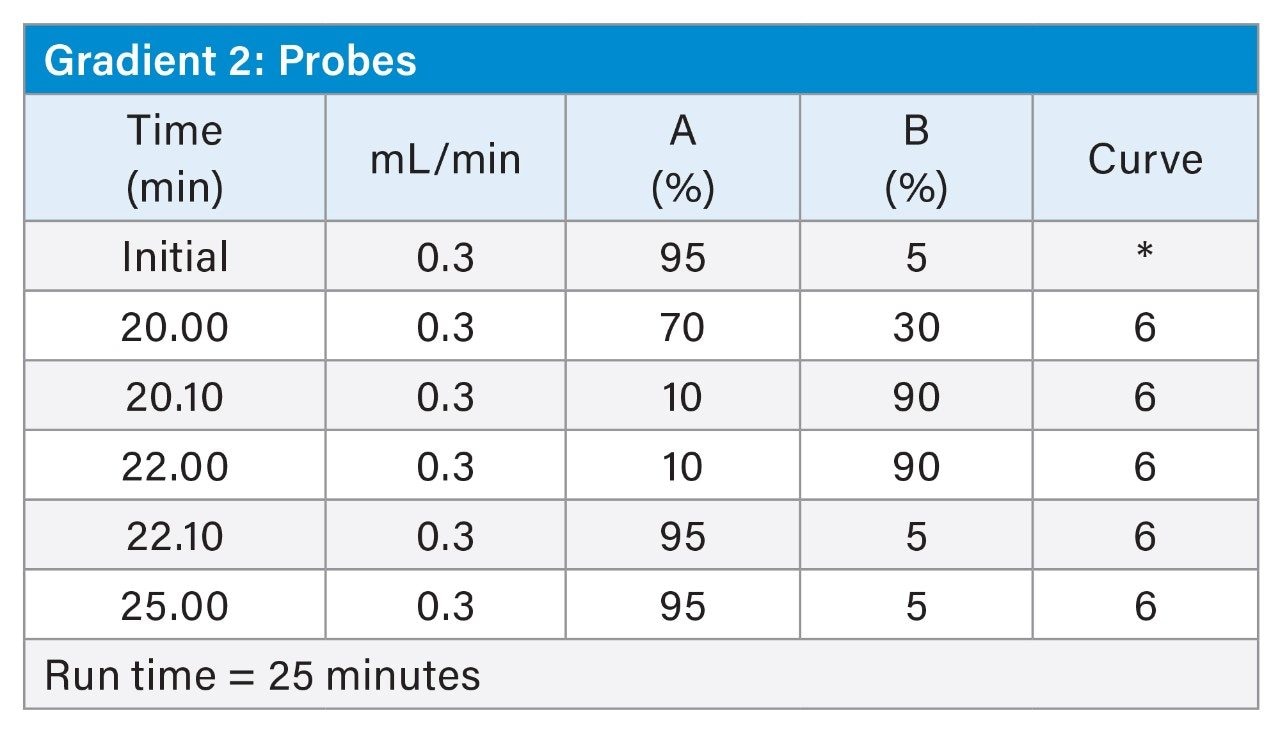
MS Conditions
|
MS system: |
BioAccord |
|
Detector: |
ACQUITY RDa Detector |
|
Mode: |
Full scan |
|
Polarity: |
Negative |
|
Cone voltage: |
40 V |
|
Mass range: |
High (400–5000 m/z) |
|
Scan rate: |
2 Hz |
|
Capillary voltage: |
0.80 kV |
|
Desolvation temperature: |
400 °C |
Results and Discussion
In ion-pairing reversed-phase chromatography (IP-RPLC), the mobile phase contains an ion pairing reagent, commonly an alkylamine, which adsorbs onto the C18 stationary phase6-8 and thereby introduces a mixed mode like retention mechanism where both oligonucleotide charge and hydrophobicity of fluorophore participate in IP-RPLC retention.6-8 The mobile phase used in this application is compatible with both optical UV detection and negative ion mode mass spectrometry.8,9
An MS-friendly mobile phase system was employed in this work that was based on using diisopropylethylamine (DIPEA) as the IP reagent along with the extra addition of 1,1,1-hexafluoroisopropanol (HFIP). HFIP has been shown to increase the sensitivity of IP-RPLC-MS analysis by improving both the retention of negatively-charged analytes to the C18 stationary phase, and the charge concentration process during electrospray ionization.6 To put this approach into practice, aqueous and acetonitrile containing mobile phases were used to develop gradients with an ACQUITY UPLC I-Class equipped with the ACQUITY UPLC TUV Detector. Oligonucleotide samples were injected at 10 pmol quantities onto an ACQUITY Premier Oligonucleotide C18 (2.1 x 50 mm, 130 Å, 1.7 µm) Column. After UV detection, the eluted oligonucleotide PCR reagents identity was verified by means of negative ion mode mass spectrometry with the ACQUITY RDa Detector of a benchtop BioAccord LC-MS System.
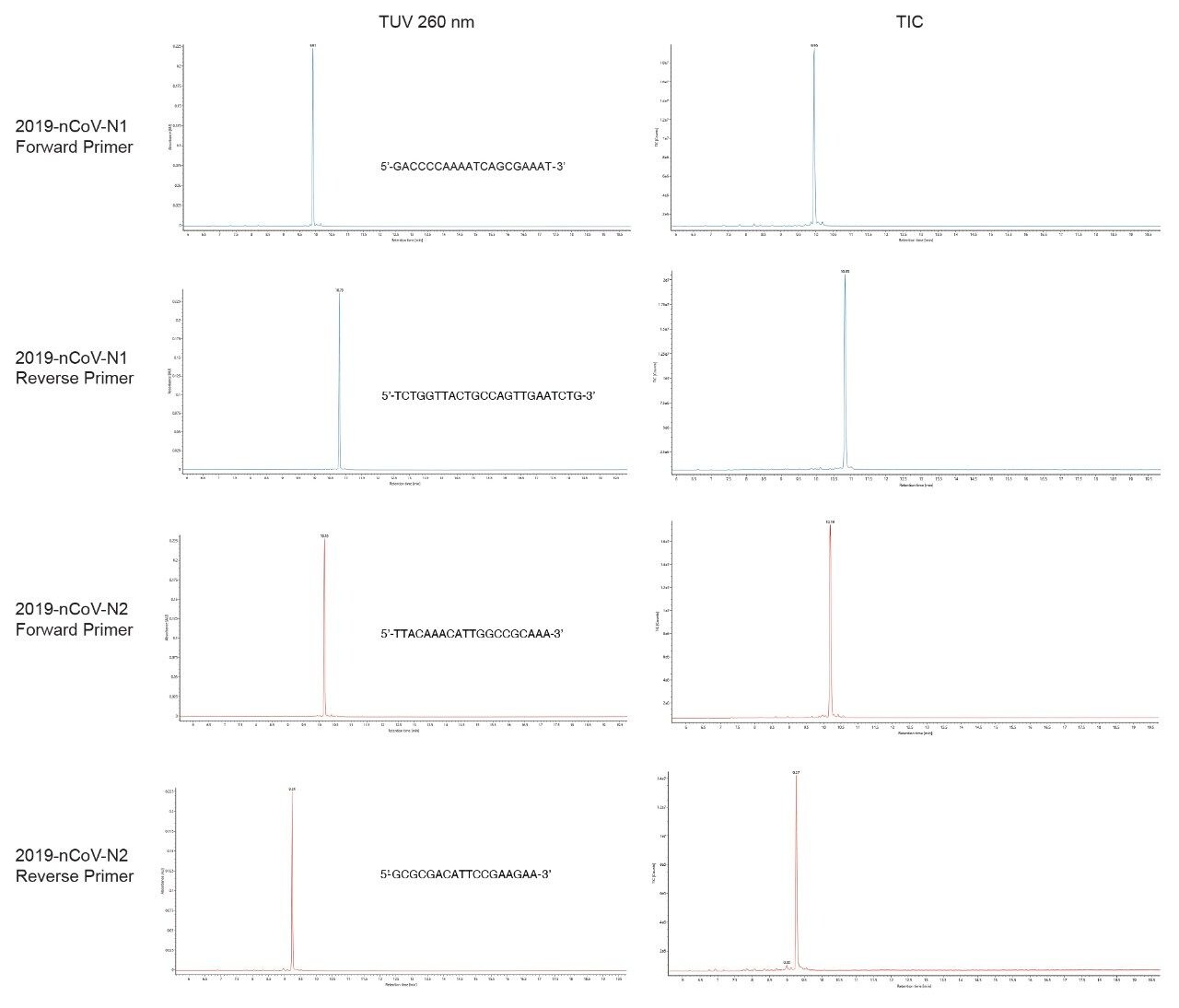
2019 SARS-CoV-2 nucleocapsid N1 and N2 primers (both forward and reverse sequences) from a CDC RT-qPCR kit were eluted from the column within 11 minutes using a 20 min gradient running from 5 to 25% B. Sharp, symmetrical peaks (Figure 1) and reproducible retention times (RT) were observed by both UV and MS chromatograms. MassPREP Oligonucleotide Standard (p/n: 186004135) ran under similar conditions, 4 months apart yielded retention times within 0.1 min reproducibility. For both N1 and N2 primers, the difference in RT between the forward and the reverse oligonucleotides was around one minute.
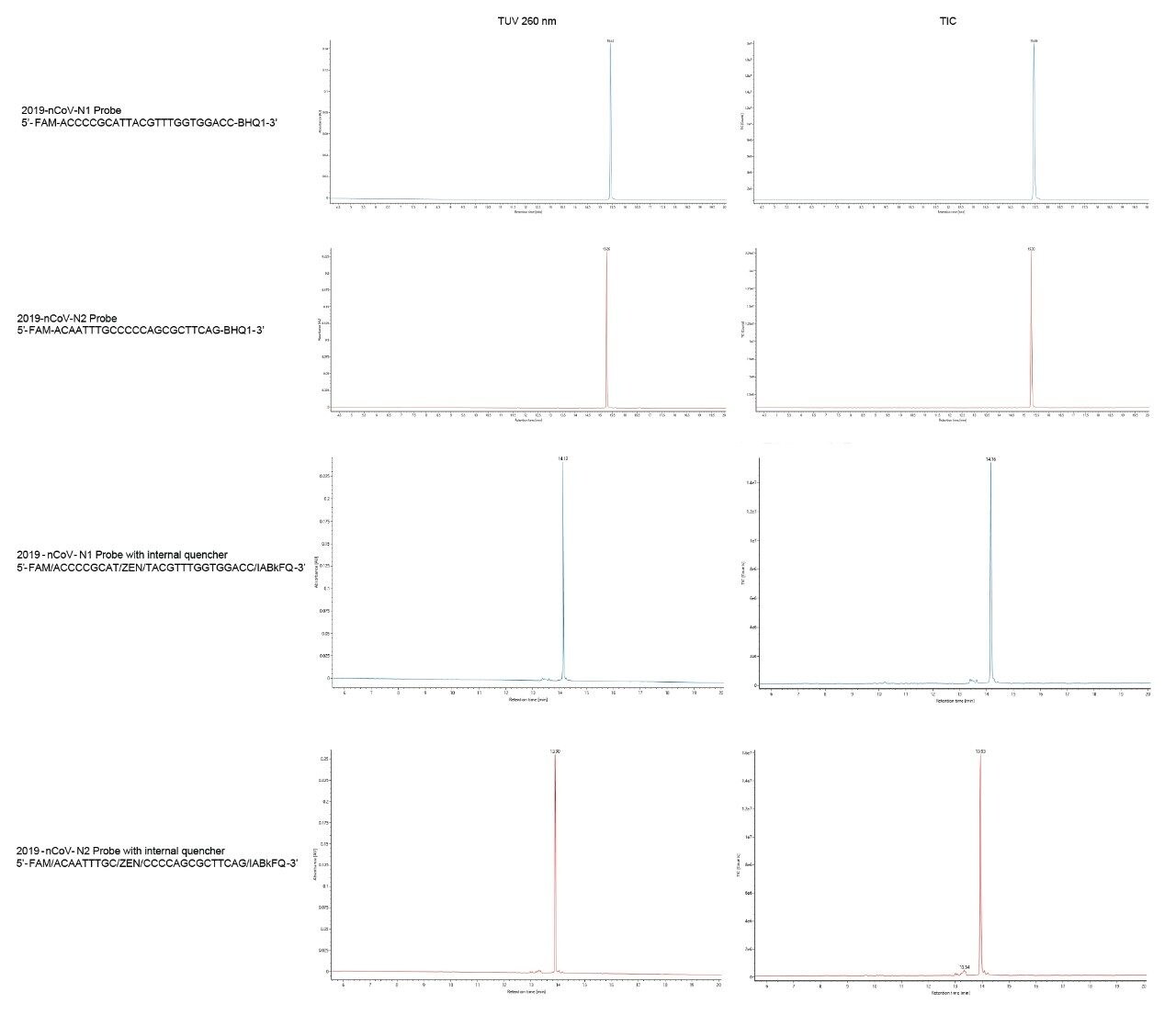
Probes used in the CDC RT-qPCR assay are labeled with a fluorophore at the 5’-end of the oligonucleotide sequence and a quencher at the 3’-end. The sensitivity of the qPCR assay is highly dependent on the proper synthesis of the fluorophore and on its attachment to the proper oligonucleotide sequence via a linker.8 Sharp peaks were also obtained for N1 and N2 probes (Figure 2). The gradient was modified to run to 30% B to better ensure elution of the more strongly retained probes. This change in final elution strength led to probes eluting at 16 versus 20-minute retention times. These results show the retention behavior of a single quencher probe. Probes that take advantage of an internal quencher, in addition to the 3’ quencher, were also analyzed. Separations achieved for these so-called double-quenched probes are shown in the bottom panel of Figure 2. Probes with an additional internal quencher eluted at an earlier retention time of about 15 minutes.
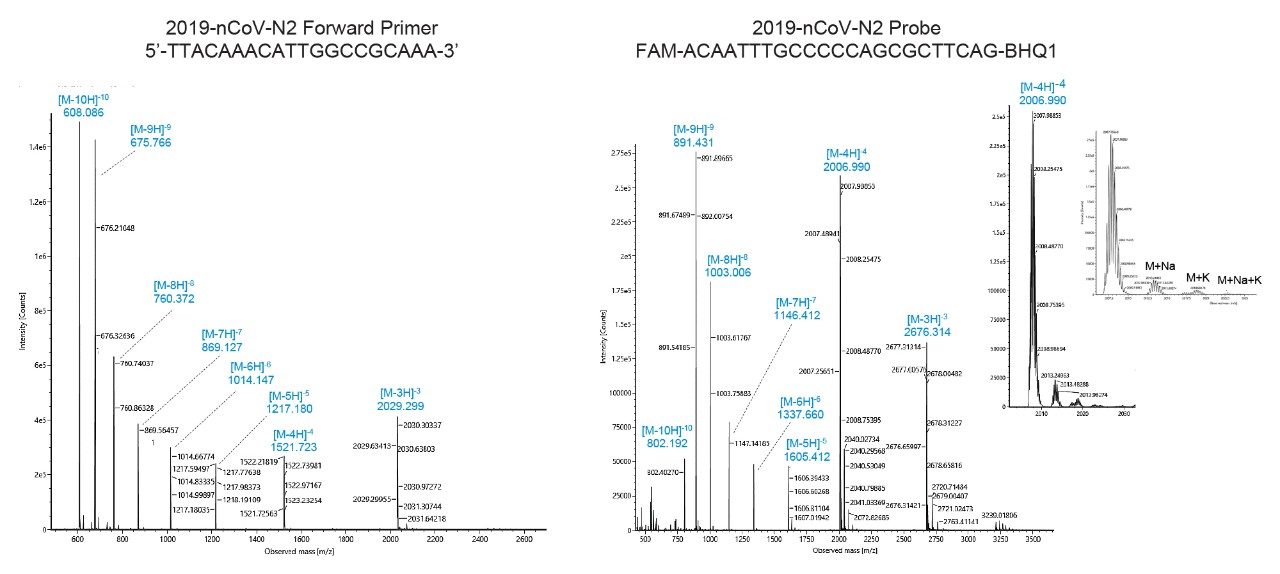
Mass spectrometric analysis with the BioAccord ACQUITY RDa Detector made mass confirmation possible. Following UV detection at 260 nm, we acquired mass spectra in negative mode between 400 and 5000 m/z at 2 Hz scan rate. Default values were used for cone and capillary voltages (40 V and 0.80 kV respectively); a desolvation temperature of 400 °C was used. The divert valve was set to MS after 1.5 minutes and back to waste at 22 minutes. Example mass spectra obtained for the N2 forward primer and N2 probe are depicted in Figure 3. Mass spectra for both primers and probes typically presented multiply charged species up to [M-10H]10-, allowing an unambiguous accurate mass determination of the RT-qPCR kit reagents (Table 2). Insets on the right panel of Figure 3 correspond to the [M-4H]4- ion of the N2 probe; sodium and potassium adducts seen on this spectrum represent less than 10% of the base peak signal. Elemental compositions and monoisotopic masses for the primers were determined based on oligonucleotide sequences using EPFL online calculator.10 Samples were analyzed in detail by HRMS yielding mass errors on accurate mass analysis of monoisotopic peaks between 0.7 and 7 ppm. The BioAccord was next applied for corresponding mass confirmation measurements. In practice, we have focused here on manual interpretation of monoisotopic peaks. However, UNIFI/waters_connect Software can be used with either MaxEnt1 or BayesSpray deconvolution algorithms to provide zero charge mass spectra. This approach to data analysis is an equally attractive means to mass analysis and mass confirmation.11,12 Molecular details about the PCR probes were proprietary; we determined the most likely molecular formula based on our accurate mass measurements using in-house Tof HRMS instrumentation followed by mass confirmation using the BioAccord LC-MS System. All mass measurements are reported in Table 1.
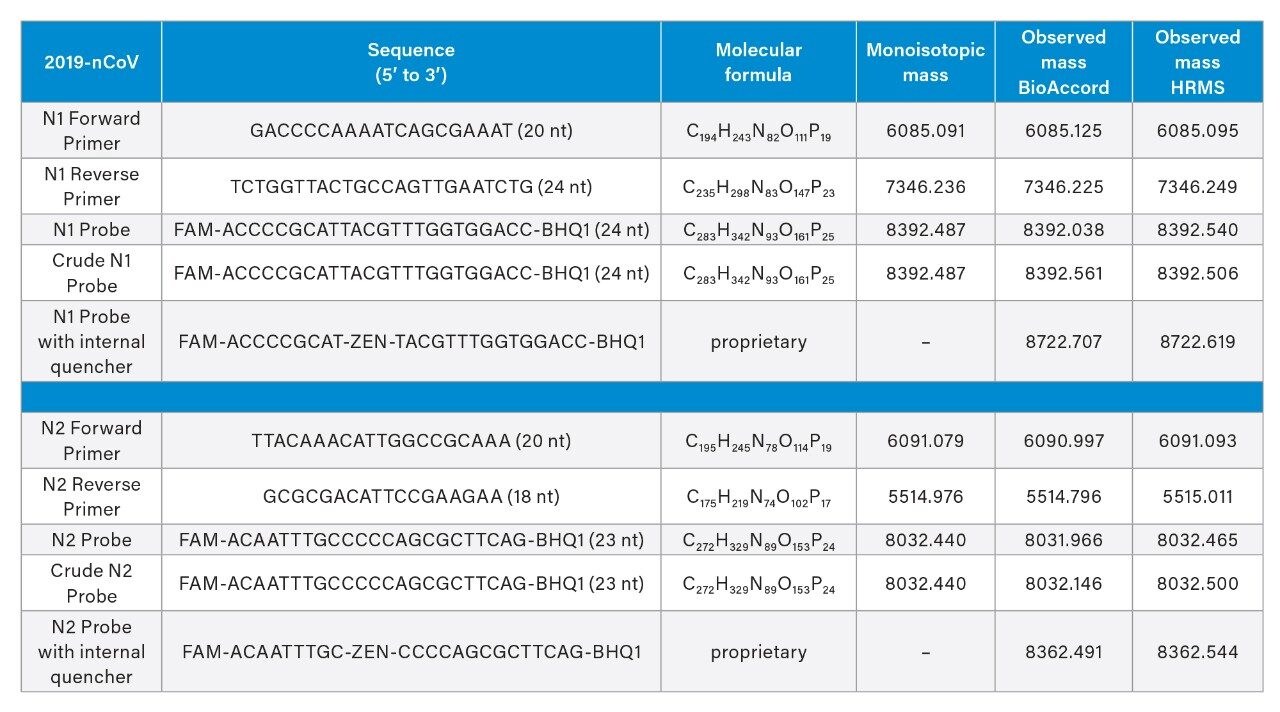
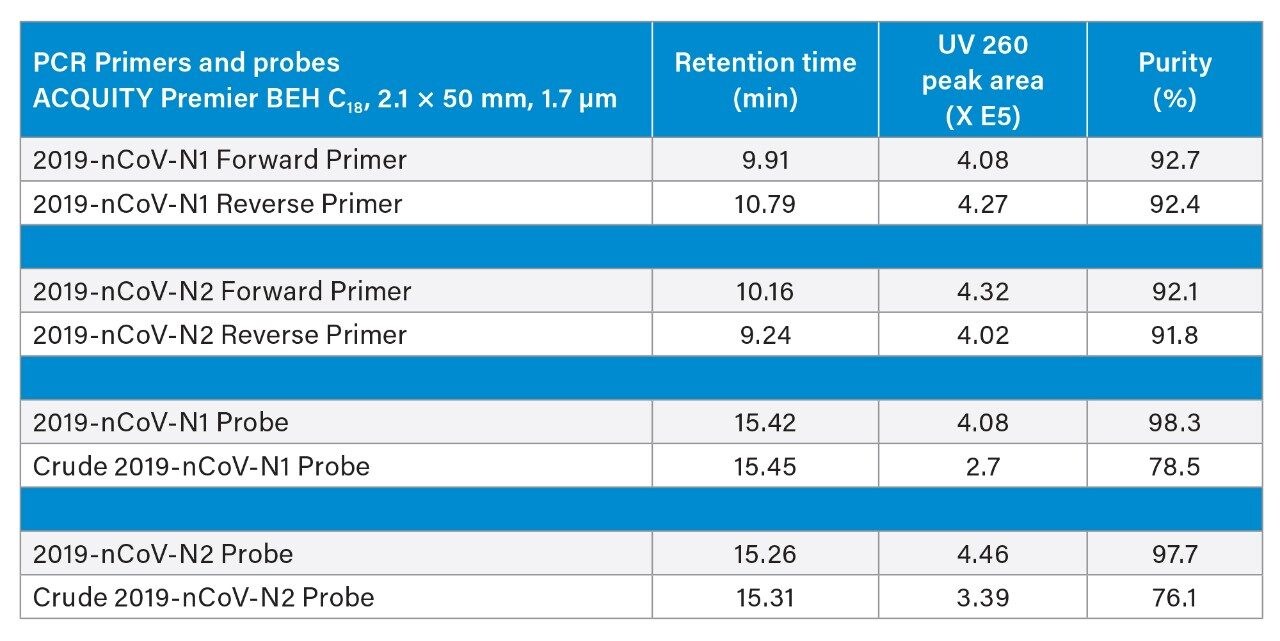
In addition to accurate mass measurements, we used IP-RPLC in combination with UV and MS to evaluate the purity of reagents and unpurified crude materials that had only been desalted after their chemical synthesis. Chromatographic peaks were therefore integrated using the collected UV chromatograms. Figure 4 displays an integrated chromatogram wherein it was of interest to calculate the percent purity of a crude N2 probe. Automatic peak integration was performed using UNIFI. Retention times, chromatographic peak area and purity are reported in Table 2. Primers qualified for the CDC assays were found to be 92% pure and their corresponding crude materials were ~90% pure. Probes qualified for the CDC assay were high purity (~98%), yet their crude (desalt only) counterparts were found to only be ~76% pure.
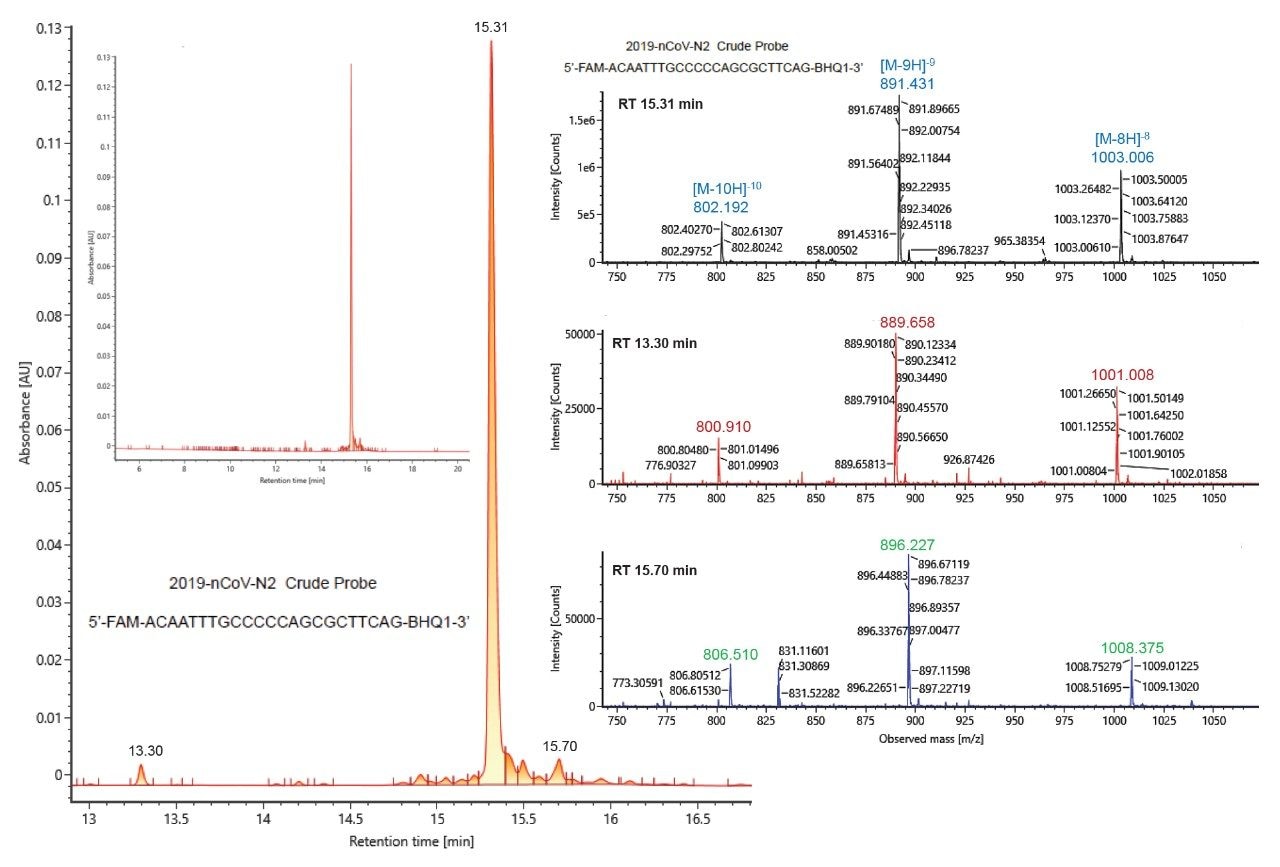
Impurities within the crude probes were examined in more detail. Along with the main chromatographic peak at 15.31 minutes, the UV trace for the crude N2 probe showed a small peak at 13.30 minutes and another at 15.70 minutes (Figure 4). Summed mass spectra obtained for these three peaks [15.31 minute (top), 13.30 minute (middle) and 15.70 min (bottom)] are depicted on the right panel of Figure 4. The 13.30 minute peak corresponds to a species with a mass shift of -16 Da when compared to the main chromatographic peak, which indicates the loss of an oxygen in the probe structure; the later eluting peak at 15.70 is a putative impurity corresponding to a positive mass shift of 42.9 Da. Altogether, data reported in Table 2 and exemplified in Figure 4 illustrate the capability of serial detection using the ACQUITY UPLC TUV Detector and the BioAccord RDa Detector for purity assessment of PCR reagents.
Conclusion
In this application note, we have described the use of optimized IP-RPLC and a compliance-ready benchtop Tof instrument for the quality assessment of PCR test reagents used in the SARS-CoV-2 CDC assay. With this sensitive approach we were able to glean significant amounts of purity information from 10 pmol quantities of nucleocapsid primers N1 and N2 (in both forward and reverse sequences) and the N1 and N2 probes. All PCR reagents yielded sharp, symmetrical peaks. Following UV detection at 260 nm, negative ion mode mass spectra were acquired and multiply charged species (up to [M-10H]10-) were readily observed. Sodium and potassium adducts represented less than 10% of the base peak signal, allowing us to unambiguously determine the molecular weights of the RT-qPCR kit reagents. The purity of reagents and unpurified crude materials were evaluated using the combination of both UV and MS detection. Automatic peak integration of UV chromatograms was performed using UNIFI for these example purity measurements. Additionally, the analytical approach described in this application note allowed for a more detailed examination of impurities as demonstrated with an investigation into the unpurified synthesis crude product of the single quencher N2 probe. In all, this methodology for combining high resolution, high sensitivity LC-UV-MS was shown to provide a deep level of information that would be of benefit to the certification of RT-qPCR reagents.
References
- WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2021 Sept 02] https://covid19.who.int/.
- Akkiz, H., Implications of the Novel Mutations in the SARS-CoV-2 Genome for Transmission, Disease Severity, and the Vaccine Development. Front. Med. 2021, 8, 10.
- Vogels, C. B. F.; Brito, A. F.; Wyllie, A. L.; Fauver, J. R.; Ott, I. M.; Kalinich, C. C.; Petrone, M. E.; Casanovas-Massana, A.; Catherine Muenker, M.; Moore, A. J.; Klein, J.; Lu, P.; Lu-Culligan, A.; Jiang, X.; Kim, D. J.; Kudo, E.; Mao, T.; Moriyama, M.; Oh, J. E.; Park, A.; Silva, J.; Song, E.; Takahashi, T.; Taura, M.; Tokuyama, M.; Venkataraman, A.; Weizman, O.-E.; Wong, P.; Yang, Y.; Cheemarla, N. R.; White, E. B.; Lapidus, S.; Earnest, R.; Geng, B.; Vijayakumar, P.; Odio, C.; Fournier, J.; Bermejo, S.; Farhadian, S.; Dela Cruz, C. S.; Iwasaki, A.; Ko, A. I.; Landry, M. L.; Foxman, E. F.; Grubaugh, N. D., Analytical Sensitivity and Efficiency Comparisons of Sars-Cov-2 RT–qPCR Primer–Probe Sets. Nature Microbiology 2020, 5 (10), 1299–1305.
- Pearson, J. D.; Trcka, D.; Lu, S. Y.; Hyduk, S. J.; Jen, M.; Aynaud, M. M.; Hernandez, J. J.; Peidis, P.; Barrios-Rodiles, M.; Chan, K.; Woodgett, J.; Mazzulli, T.; Attisano, L.; Pelletier, L.; Cybulsky, M. I.; Wrana, J. L.; Bremner, R., Comparison of SARS-CoV-2 Indirect and Direct RT-qPCR Detection Methods. Virol. J. 2021, 18 (1), 12.
- Filges, S.; Mouhanna, P.; Ståhlberg, A., Digital Quantification of Chemical Oligonucleotide Synthesis Errors. Clinical Chemistry 2021.
- Guo, L.; Worth, A. J.; Mesaros, C.; Snyder, N. W.; Glickson, J. D.; Blair, I. A., Diisopropylethylamine/Hexafluoroisopropanol-Mediated Ion-Pairing Ultra-High-Performance Liquid Chromatography/Mass Spectrometry for Phosphate and Carboxylate Metabolite Analysis: Utility for Studying Cellular Metabolism. Rapid Commun Mass Spectrom 2016, 30 (16), 1835–1845.
- Birdsall, R. E.; Gilar, M.; Shion, H.; Yu, Y. Q.; Chen, W., Reduction of Metal Adducts in Oligonucleotide Mass Spectra in Ion-Pair Reversed-Phase Chromatography/Mass Spectrometry Analysis. Rapid Commun Mass Spectrom 2016, 30 (14), 1667–1679.
- Fountain, K.; Gilar, M.; Budman, Y.; Gebler, J., Purification of Dye-Labeled Oligonucleotides by Ion-Pair Reversed-Phase High-Performance Liquid Chromatography. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 2003, 783, 61–72.
- Jiang, T.; Yu, N.; Kim, J.; Murgo, J.-R.; Kissai, M.; Ravichandran, K.; Miracco, E. J.; Presnyak, V.; Hua, S., Oligonucleotide Sequence Mapping of Large Therapeutic mRNAs via Parallel Ribonuclease Digestions and LC-MS/MS. Anal. Chem. 2019, 91 (13), 8500–8506.
- Ortiz, D.; Gasilova, N.; Sepulveda, F.; Patiny, L.; Dyson, P. J.; Menin, L., Aom2S: A New Web-Based Application for DNA/RNA Tandem Mass Spectrometry Data Interpretation. Rapid Communications in Mass Spectrometry 2020, 34 (23), e8927.
720007429, December 2021