A modernized version of the USP method for organic impurities in cetirizine HCl tablets has been demonstrated on four different LC systems from two different vendors. Similar results were obtained on the HPLC and UHPLC systems used. These experiments were all performed using the same XBridge HILIC Column, providing confidence that validated methods can be successfully transferred between different LC systems.
Cetirizine is a second-generation antihistamine that is used in the treatment of hay fever, urticaria, angioedema, and allergies. The USP method for organic impurities in cetirizine hydrochloride tablets specifies the use of a 4.0 x 250 mm, 5 μm L3 column (porous silica particles, 1.5 μm–10 μm diameter, or a monolithic rod).1 Permissible alterations are given in USP General Chapter <621>, according to which, for isocratic methods, particle size (dp) and/or column length (L) can be changed as long as the ratio of column length to particle size (L/dp) remains constant or within the range -25% to +50% of the original column specified. Alternatively, other combinations of L and dp may be employed provided the number of theoretical plates (N) is within the range -25% to +50% of the original column specified in the method.2
Hence, an existing USP method can be modernized by using newer column chemistries without the need to revalidate the method. Columns containing smaller particles substantially reduce analysis times without compromising the quality of the data, when the flow rate is scaled accordingly. The organic impurity method for cetirizine hydrochloride tablets has been updated by using an XBridge HILIC XP, 2.5 μm, 4.6 x 100 mm Column (p/n: 186006087).3 The USP allowable changes reduced the analysis time by a factor of 5 (from 15 minutes to 3 minutes) and improved the peak shape for cetirizine HCl. The peak shape improvement was due to injecting a scaled, smaller injection volume, which mitigated much of the peak distortion caused by the sample diluent. Further evidence of sample diluent induced peak distortion will be presented in this work.
Also Run On:
Agilent 1100 Binary System
Agilent 1260 Infinity Quaternary System
Agilent 1290 Infinity Quaternary System
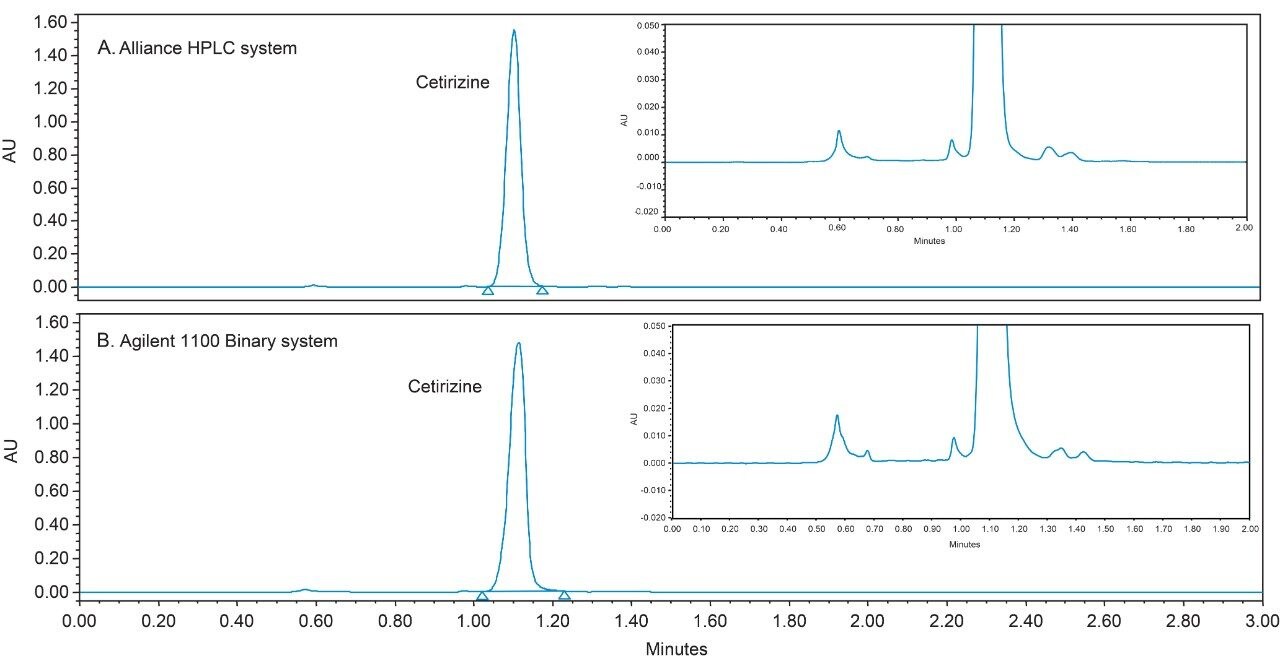
USP methods are validated and are generally accepted to work on any properly functioning LC system. However, few examples of successful HILIC transfers between systems from different vendors have been shown. The same HILIC method and column were tested on four different LC systems, from Waters (Alliance HPLC System) and Agilent (1100 Binary HPLC System, 1260 Infinity Quaternary UHPLC System and 1290 Infinity Quaternary UHPLC System), each controlled by Empower CDS. The particular method used required some investigation, in which a key obstacle was overcome to achieve undistorted peaks.
The method was then modified using a MS-compatible mobile phase to demonstrate the feasibility of further modernizing the method.
Generic cetirizine tablets (10 mg) were used in this study and were prepared as per the USP cetirizine hydrochloride tablets organic impurity method. Five tablets were crushed and the powder was transferred to a 100 mL volumetric flask containing about 50 mL of diluent (910:27:63 acetonitrile: solution A [2:33 2 N sulfuric acid: water]: water), sonicated for about 20 minutes and diluted to the volume mark with diluent, to get a concentration of 0.5 mg/mL. The sample was filtered with a 0.45 μm GHP Acrodisc filter into LCMS Certified Vials.
To troubleshoot the poor peak shape observed when carrying out the USP method, the sample was prepared in three different diluents, listed below. Five cetirizine hydrochloride (10 mg) tablets were crushed and the powder was transferred to a 100 mL volumetric flask for each preparation. About 50 mL of diluent was added to each and the flask was sonicated for about 20 minutes. The solutions were diluted to the volume mark with diluent, to arrive at a concentration of 0.5 mg/mL, and mixed well. Before injecting in the LC system the sample was filtered with a 0.45 μm GHP Acrodisc filter into LCMS Certified Vials.
Five cetirizine hydrochloride tablets were crushed and the powder was transferred to a 100 mL volumetric flask. About 50 mL of mobile phase (93:7 acetonitrile: 200 mM ammonium formate buffer) was added and the flask was sonicated for about 20 minutes. The solution was diluted to volume with mobile phase to get a concentration of 0.5 mg/mL and mixed well. Before injecting the sample, the sample solution was filtered through a 0.45 μm GHP Acrodisc filter into LCMS Certified Vials.
|
LC systems: |
Alliance HPLC System with 2489 UV/Visible Detector |
|
Agilent 1100 Binary LC System with Agilent 1100 DAD Detector |
|
|
Agilent 1260 Infinity Quaternary LC System with Agilent 1260 DAD Detector |
|
|
Agilent 1290 Infinity Quaternary LC System with Agilent 1290 DAD Detector |
|
|
Column: |
XBridge HILIC XP, 2.5 μm, 4.6 x 100 mm |
|
(p/n: 186006087) |
|
|
Column temp.: |
25 °C |
|
Injection volume: |
10.6 μL |
|
Flow rate: |
2.116 mL/min |
|
Separation mode: |
Isocratic |
|
Solution A: |
2:33 2 N sulfuric acid: water |
|
Buffer solution: |
3.4 g/L tetrabutyl ammonium hydrogen sulfate in water |
|
Mobile phase: |
93:5:2 acetonitrile: solution A: buffer solution |
|
LC system: |
Alliance e2695 HPLC |
|
Column temp.: |
25 °C |
|
Injection volume: |
4 μL |
|
Flow rate: |
1.0 mL/min |
|
Mobile phase: |
93:7 acetonitrile: 200 mM ammonium formate buffer (pH 2.9, adjusted with formic acid) |
|
Mass spectrometer: |
ACQUITY QDa |
|
Vials: |
LCMS Certified – clear, preslit (p/n: 600000668CV) |
Empower 3 CDS
The USP organic impurities method for cetirizine hydrochloride was scaled down from a 4.0 x 250 mm, 5 μm to a 4.6 x 100 mm, 2.5 μm column using conditions obtained from the Waters’ column calculator and run on an Alliance e2695 HPLC System with a 2489 UV/Visible Detector. The USP system suitability criteria of tailing factor NMT 2.0 and relative standard deviation (RSD) NMT 10.0% were met. Average USP tailing for six replicate injections of cetirizine was 1.3 and % RSD was found to be 0.8% for peak area.
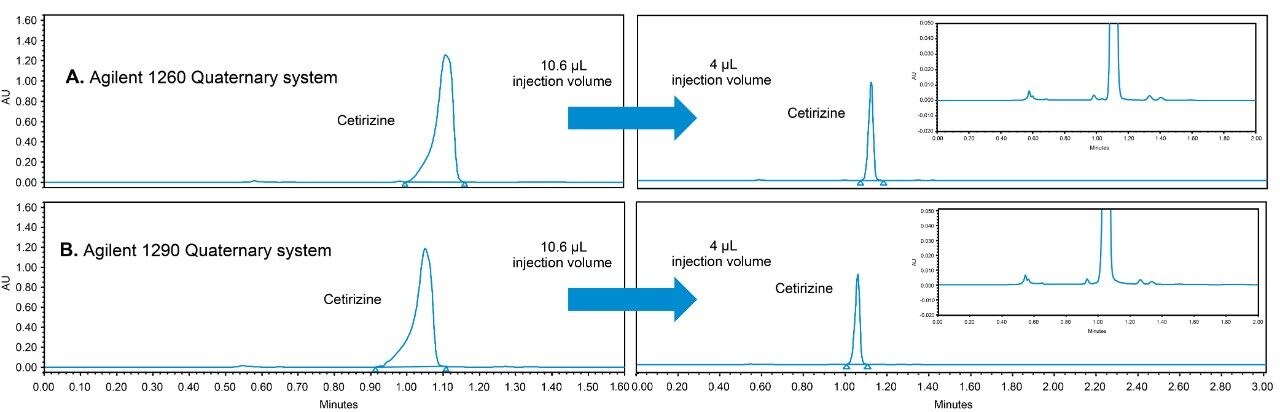
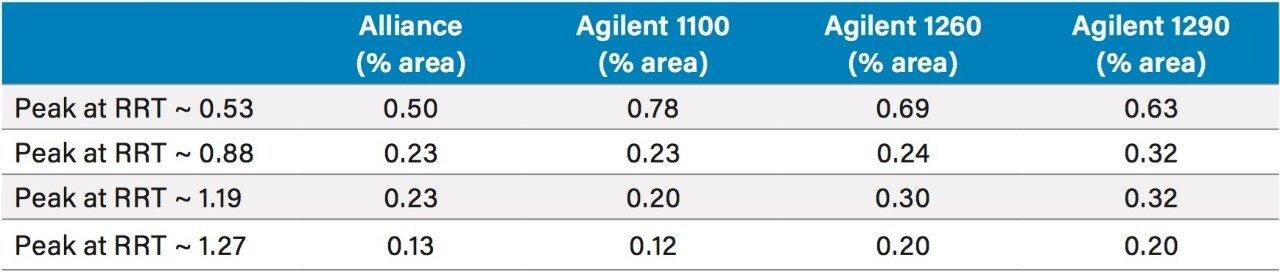
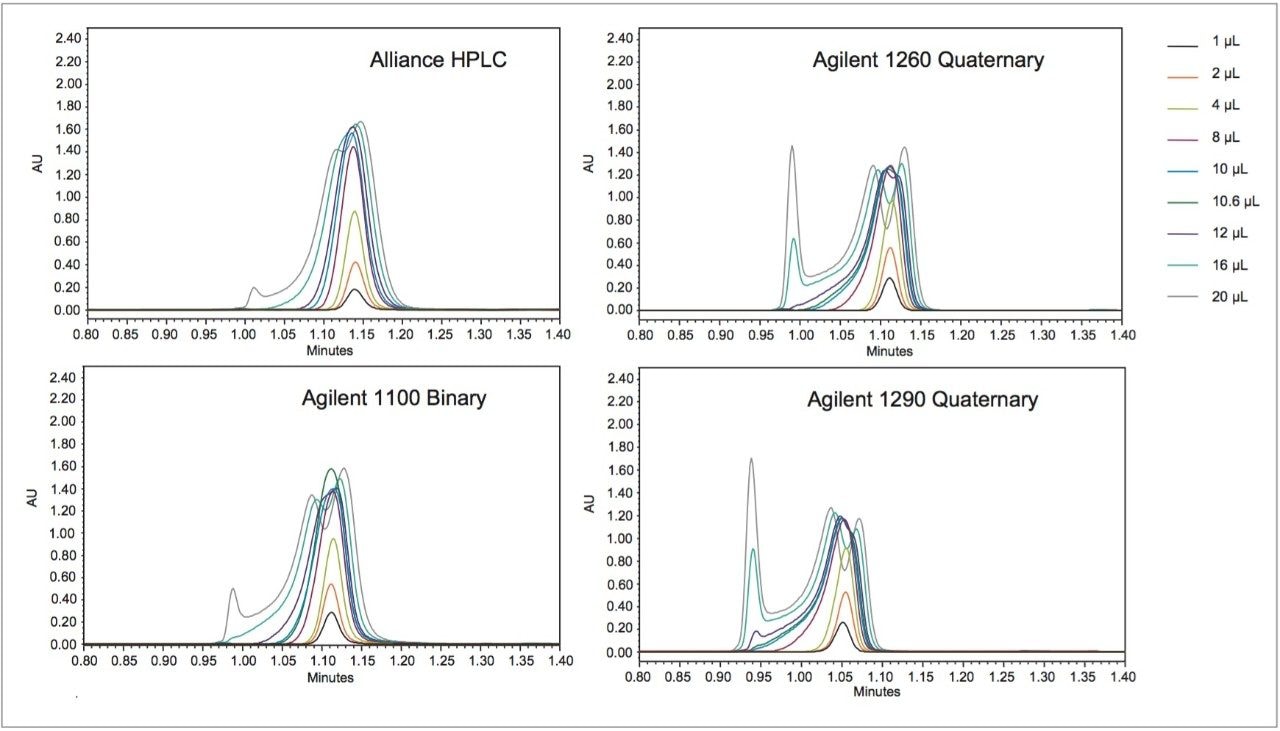
The same mobile phase, column, and sample were used on four different LC systems with various injection volumes. According to USP guidelines, the injection volume can be adjusted, provided all the required criteria are met. In this case, sufficient sensitivity of organic impurities is necessary.4 The results obtained were reproducible on the HPLC instruments as shown in Figure 1 and on the UHPLC instruments as shown in Figure 2. The smaller impurity peaks observed were also reproducible and the % areas were comparable on each instrument as shown in Table 1. As expected, the retention times are comparable, with some variation, likely due to system volume differences or other effects. For the scaled injection volume (10.6 μL injected; USP method calls for 20 μL), the peak shape for cetirizine is noticeably different on each system. The peak fronting is most noticeable on the Agilent 1260 and Agilent 1290 UHPLC systems, but improved peak shapes are observed at a lower injection volume (4 μL), as shown in Figure 2. This is because these two systems, as configured, have the lowest dispersion of the four systems tested. The relatively larger dispersion of the Agilent 1100 and Waters Alliance HPLC Systems mitigate some of the peak distortion by reducing the strong solvent effects, i.e. mixing the injected sample plug with the mobile phase before the column inlet. In fact, adding additional tubing volume between the injector and column inlet has been demonstrated as a tactic to mitigate strong solvent effects in liquid chromatography, but at the cost of apparent column efficiency.5 Increasing injection volumes are compared in Figure 3 for each LC system used. In all cases, the lowest injection volume, 1 μL, shows very good peak shape, which becomes distorted as the injection volume increases. As the injection volume is increased above 4 μL (for example 8, 10, 10.6, 12 μL…), the peak for cetirizine begins to front significantly and even split into two or three peaks. With the given method, adjusting the injection volume would be necessary to observe acceptable peak shape on each instrument, but success can be achieved nonetheless.
To further investigate the peak distortion observed, the sample was prepared in the USP specified mobile phase, which consisted of 93% acetonitrile and 7% aqueous solution. The sample prepared using mobile phase as the solvent was injected at volumes of 1, 2, 4, 8, 10, 10.6, 12, 16, and 20, 30, and 40 μL. In all cases, dissolving cetirizine in the mobile phase produced ideal, Gaussian peak shapes, the peak height and width growing proportionally, even at the highest injection volumes, until the UV detector signal was saturated (demonstrated on Alliance HPLC System in Figure 4B). This led to the following two hypotheses for the cause of the observed peak distortion:
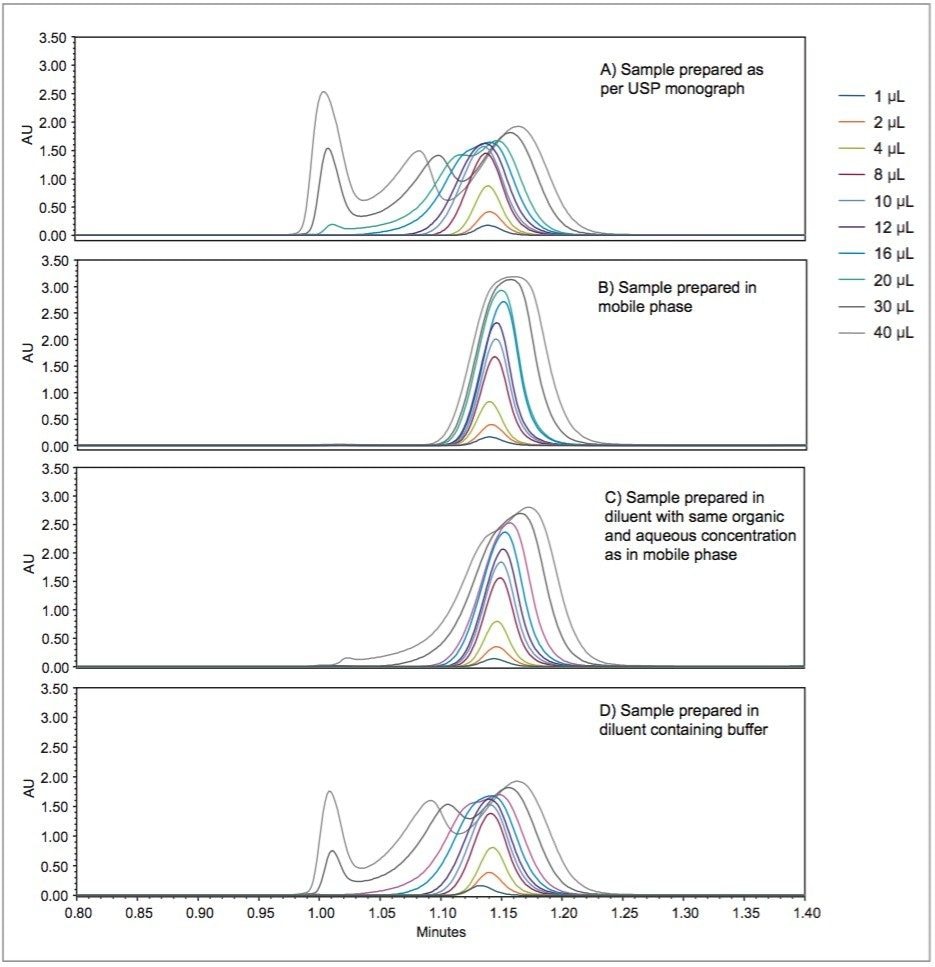
These experiments lead us to conclude that the distortions in peak shape observed are due mostly to the imbalance between the organic and aqueous concentrations in the mobile phase and sample solvent, causing an obviously strong solvent effect. The absence of buffer appears to have only a minor effect, slightly distorting the peak of cetirizine. Matching both the aqueous content and the buffer concentration in combination helps in obtaining a Gaussian peak at all injection volumes, hence the exceptional results when dissolving the sample in the mobile phase.
Finally, some experimental proof was generated to transfer the USP method, which utilizes tetrabutyl ammonium hydrogen sulfate as buffer, to a MS compatible buffer, ammonium formate (pH 2.9, adjusted with formic acid). The use of MS detection can improve the quality of data collected (confirmation of cetirizine and impurities by mass and peak purity). The same XBridge HILIC XP, 2.5 μm, 4.6 x 100 mm Column (p/n: 186006087) used in the previous sections was used in these experiments. The flow rate was reduced from 2.116 mL/min to 1 mL/min to accommodate the ESI interface, which results in longer separation time. In some cases, it may be advantageous to redevelop an LC method using another mode of chromatography, such as reversed-phase; however, we elected to maintain a HILIC method to demonstrate transfer of the method to a MS-compatible mobile phase on the same chromatographic system without the need to change the column.
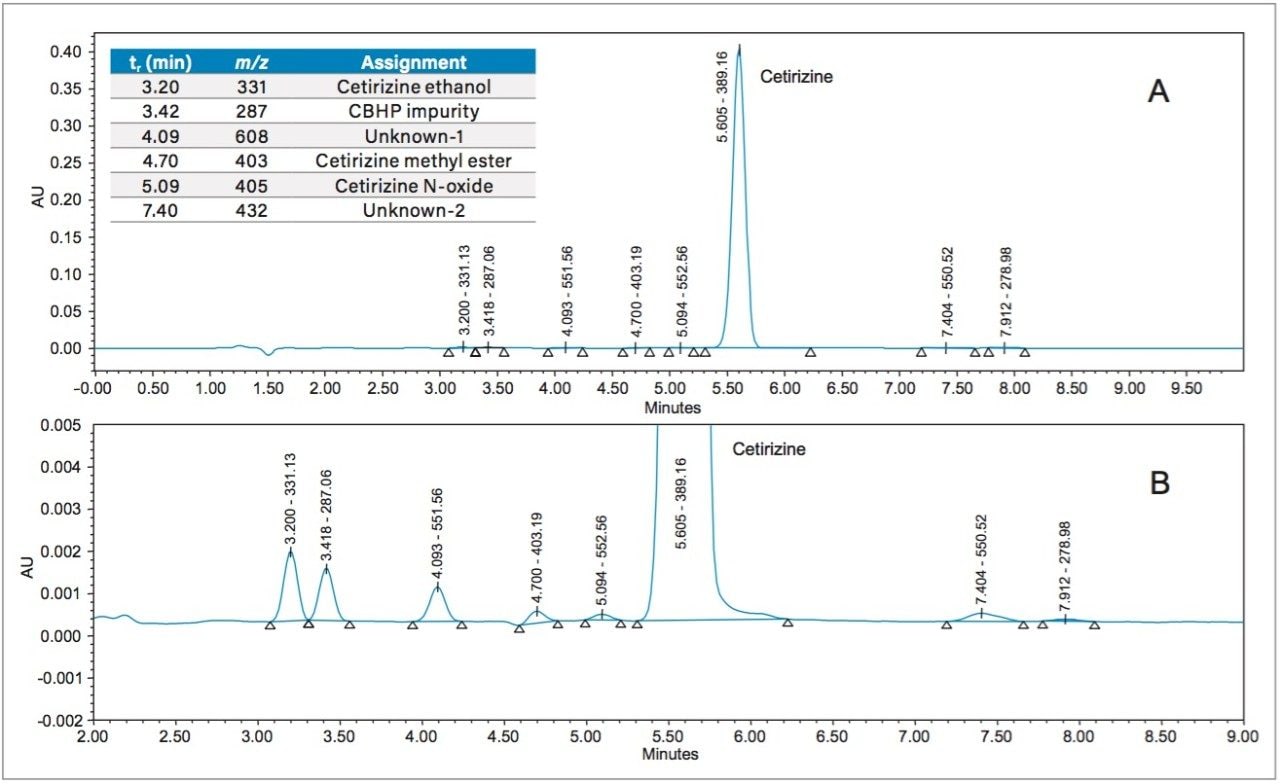
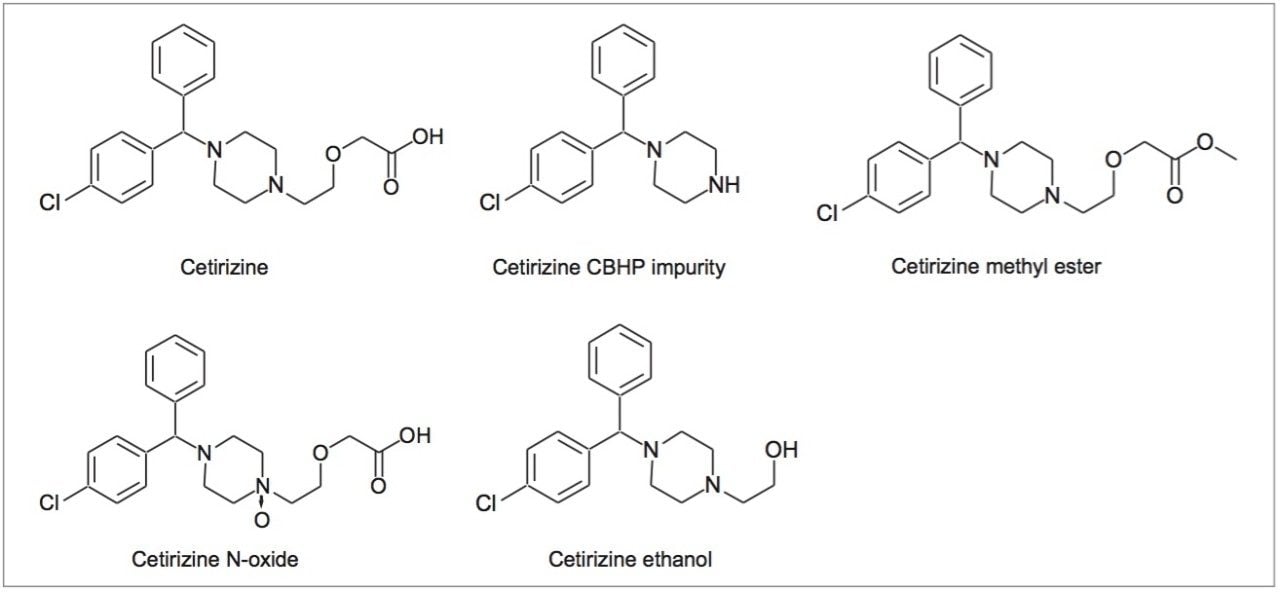
The sample was prepared in mobile phase, the best practice to achieve undistorted peaks, and an ACQUITY QDa Mass Detector was plumbed after the UV detector (Waters Alliance HPLC controlled by Empower 3 FR3). The chromatogram of a 4 μL injection of sample is shown in Figure 5. The change in buffer causes some selectivity changes, as expected, compared with the ion pairing buffer specified in the USP method. When integrating the peaks, the UV peaks are cross referenced with the most intense m/z at the same retention time (the retention volume offset must be accounted for to get proper alignment of the chromatograms). Of the two impurities identified in the USP monograph, only the cetirizine ethanol impurity was detected, while the cetirizine lactose ester impurity was not. Three other impurities, previously reported in cetirizine tablets,6,7 were tentatively identified based on the most intense m/z values. The proposed LC-MS method would meet the USP criteria for tailing factor and retention time reproducibility.
A modernized version of the USP method for organic impurities in cetirizine HCl tablets has been demonstrated on four different LC systems from two different vendors. Similar results were obtained on the HPLC and UHPLC systems used. These experiments were all performed using the same XBridge HILIC Column, providing confidence that validated methods can be successfully transferred between different LC systems. This USP method has been shown to have a significant issue caused by the difference between the sample solvent and the mobile phase. The work presented here shows that the largest contributor to the observed peak distortion is the higher percentage of strong eluent (water in the case of the prescribed HILIC method) in the sample solvent than in the mobile phase, 9% vs. 7%, respectively. While the offset in aqueous content may seem small, it causes severe distortion of the cetirizine peak at high injection volumes. It is hypothesized that the water injected from the sample solvent has a retention factor similar to that of cetirizine under these conditions. If the bands of water and cetirizine move through the column bed at approximately the same speed, the peak shape will be continually distorted until elution. Additional work to test this hypothesis is underway.
720005870, February 2017