Quantification of matrix factor is required for bioanalytical methods in support of pre-clinical and clinical studies, as the endogenous compounds in blood-derived products can cause ion suppression or enhancement leading to inaccurate results. In this application note, we describe the use of UNIFI software used with the ACQUITY UPLC I-Class/Xevo TQ-S systems for automated determination of the matrix factor for a bioanalytical assay of fluticasone propionate in plasma.
The Waters Regulated Bioanalysis System Solution features workflow-driven software that controls both UPLC and MS instruments and provides advanced data management capabilities for regulated laboratories. The solution can be used to automatically determine the matrix factors of a bioanalytical assay.
In the field of quantitative bioanalysis, LC-MS/MS is the analytical technique of choice due to its high sensitivity and selectivity. However, quantitative bioanalytical methods development by LC-MS/MS can be complicated by matrix-derived interferences such as phospholipids. Matrix factor calculation has been the subject of much discussion in bioanalytical meetings, resulting in guidelines on its evaluations being recommended at the Crystal City conferences.
As a part of the method validation process in bioanalysis, it is a requirement that ion suppression due to matrix effect be determined. However, the matrix factor calculation process can be time-consuming and tedious, requiring transfer of data to and from external software programs for advanced processing, a process which is often not 21 CFR Part 11 compliant.
The Waters Regulated Bioanalysis System, featuring UNIFI informatics and data management, enables bioanalytical laboratories to acquire and process LC-MS/MS-derived information in a streamlined and compliant-ready workflow. In this application note, we describe the use of UNIFI software used with the ACQUITY UPLC I-Class/Xevo TQ-S systems for automated determination of the matrix factor for a bioanalytical assay of fluticasone propionate in plasma.
Plasma proteins were precipitated using acetonitrile
|
LC system: |
ACQUITY UPLC I-Class System |
|
LC column: |
ACQUITY UPLC BEH C18 Column, 1.7 μm, 2.1 x 50 mm |
|
Column temp.: |
45 °C |
|
Sample temp.: |
4 °C |
|
Injection vol: |
5 μL |
|
Flow rate: |
600 μL/min |
|
Mobile phase A: |
0.1% ammonium hydroxide in water |
|
Mobile phase B: |
Methanol |
|
Gradient 1: |
5 to 95% B in 1 min |
|
Gradient 2: |
5 to 95% B in 2 min |
|
MS system: |
Xevo TQ-S, ESI positive mode |
|
Capillary voltage: |
1.0 kV |
|
Cone voltage: |
18 V |
|
Collision energy: |
20 eV |
UNIFI Scientific Information System
The matrix factor calculation performed using the UNIFI Scientific Information System follows the workflow detailed in Figure 1. Following this protocol, the matrix factor is calculated by comparing LC-MS/MS signal response for the analyte in solvent, and blank matrix samples.
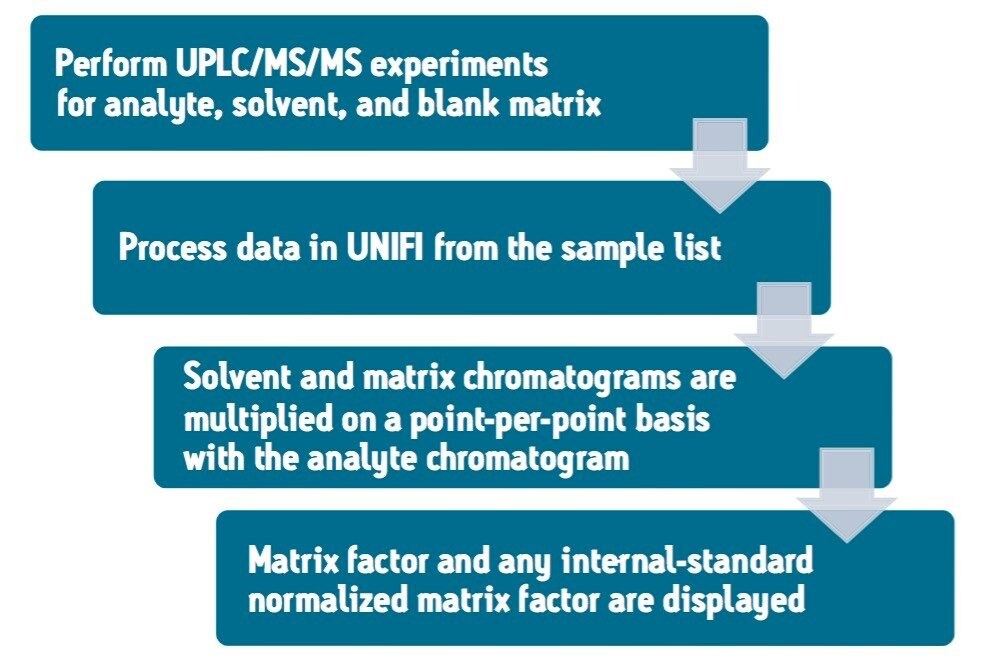
Fluticasone propionate was analyzed in plasma following protein precipitation with acetonitrile. Two sets of experimental conditions were used, a short gradient of 1 min and a longer gradient of 2 min. The matrix factor was determined for different gradient profiles (1 and 2 min). The elution profile for fluticasone propionate was determined for each chromatographic method. Fluticasone propionate and its D3 internal standard were infused into the LC stream post-column for both the solvent blank and matrix blank. This process was performed under complete automation using the on-board fluidics of the Xevo TQ-S, eliminating the need for time consuming re-plumbing of the system.
UNIFI software was used to control the UPLC and MS instruments and also to process the data, including automatically calculating the matrix factor for all experiments.
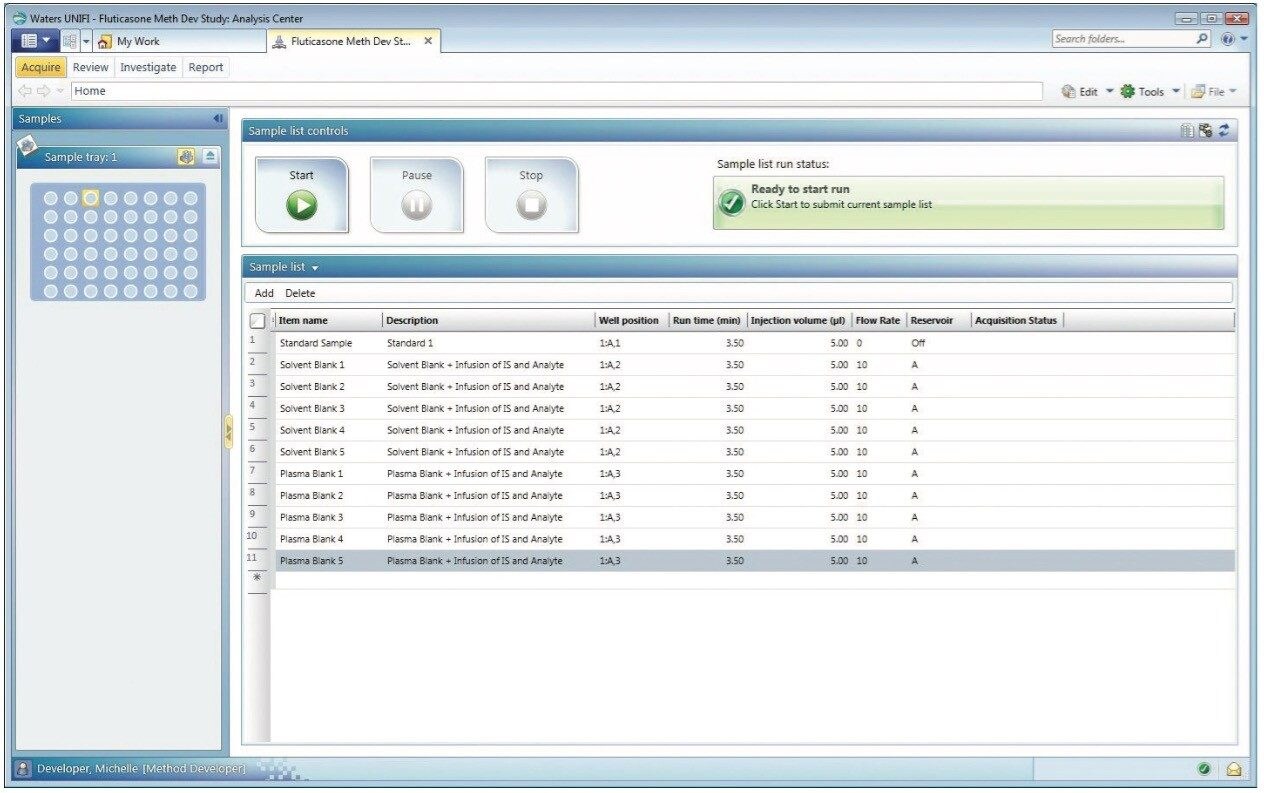
Figure 2 shows the sample list along with the well position, run time (min), injection volume (μL), flow rate, and the solvent reservoir for each sample in UNIFI software.. The left panel of the figure shows the particular position of the selected sample (plasma blank 5 that is infused with internal standard and analyte) in the Sample Manager.
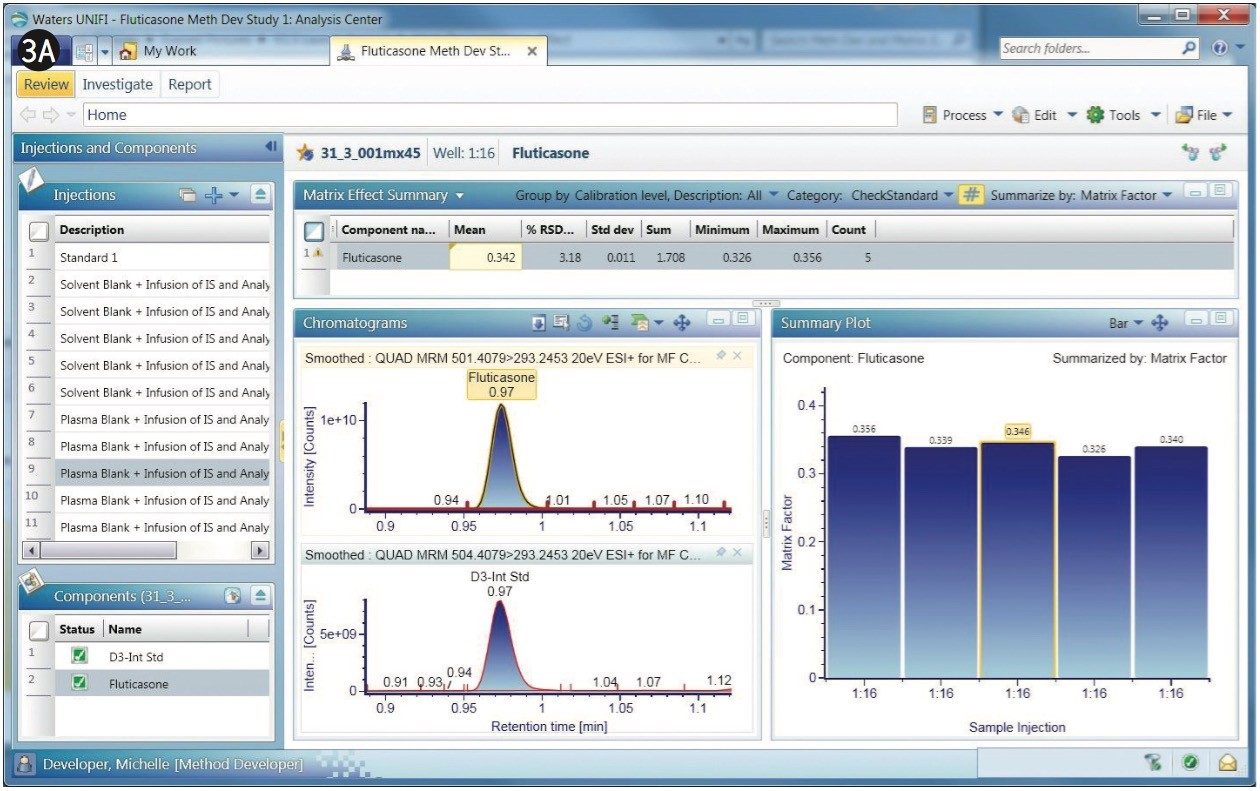
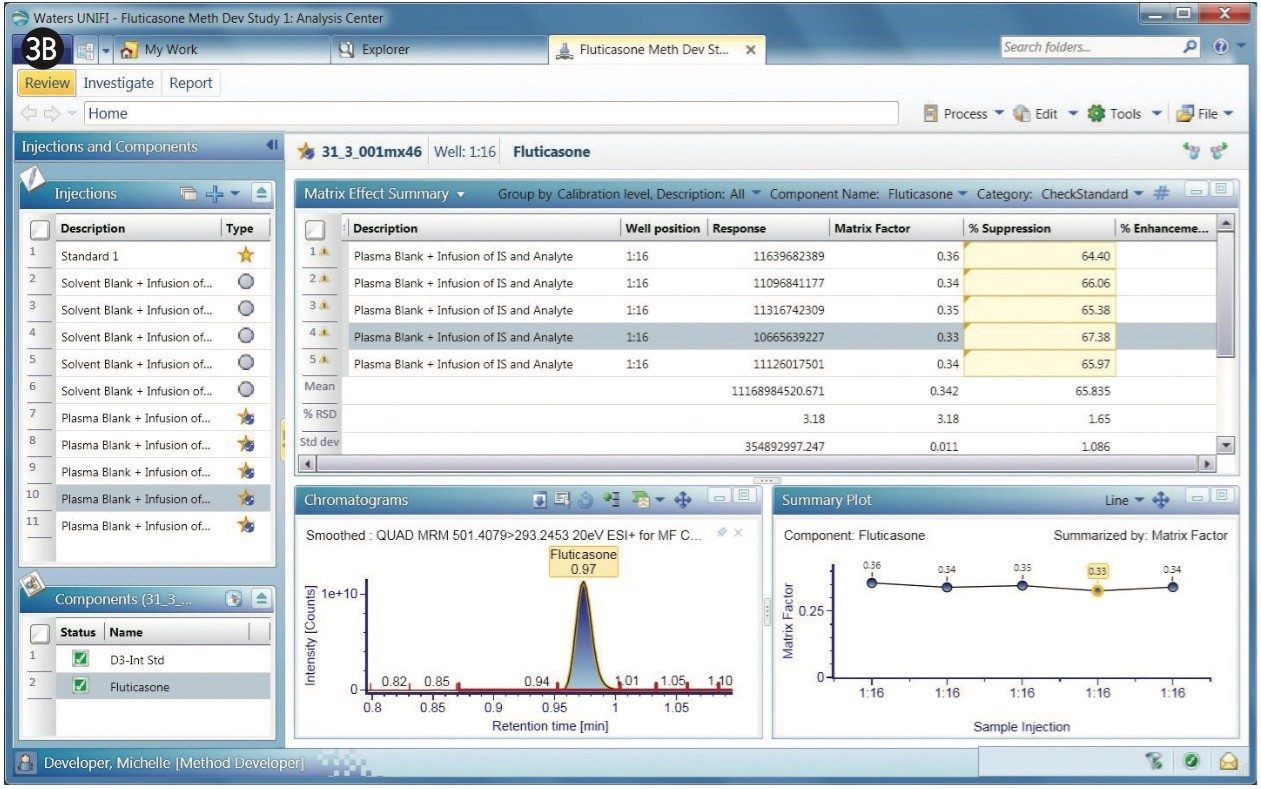
The chromatograms of fluticasone propionate with a 1-min gradient are shown in Figures 3A and 3B. After the experiments are performed, any given sample from the sample list can be selected to display its chromatogram. Figure 3A displays a comparison of MRM of the fluticasone propionate with that of the D3 internal standard. As shown, fluticasone propionate has a retention time of 0.97 min.
Fluticasone elutes in a region of the chromatogram where there appears to be severe matrix suppression. This suppression is reflected in the matrix factor, which is calculated at 0.35, illustrated in the bar chart within Figure 3A. Here, UNIFI software displays the summary plot of matrix factor for five injections of fluticasone propionate in the form of a histogram. Additionally, the summary plot can be represented in graphical mode, as shown in Figure 3B, showing the sample list displayed along with response, matrix factor, and percent suppression. The % CV for the matrix factor is also displayed in the summary.
Similar to the data shown in Figures 3A and 3B, the MRMs, matrix factors, and sample list are shown for the fluticasone propionate with a 2-min gradient (Figure 4). The retention time of the fluticasone propionate was determined to be 1.95 min for the 2-min gradient.

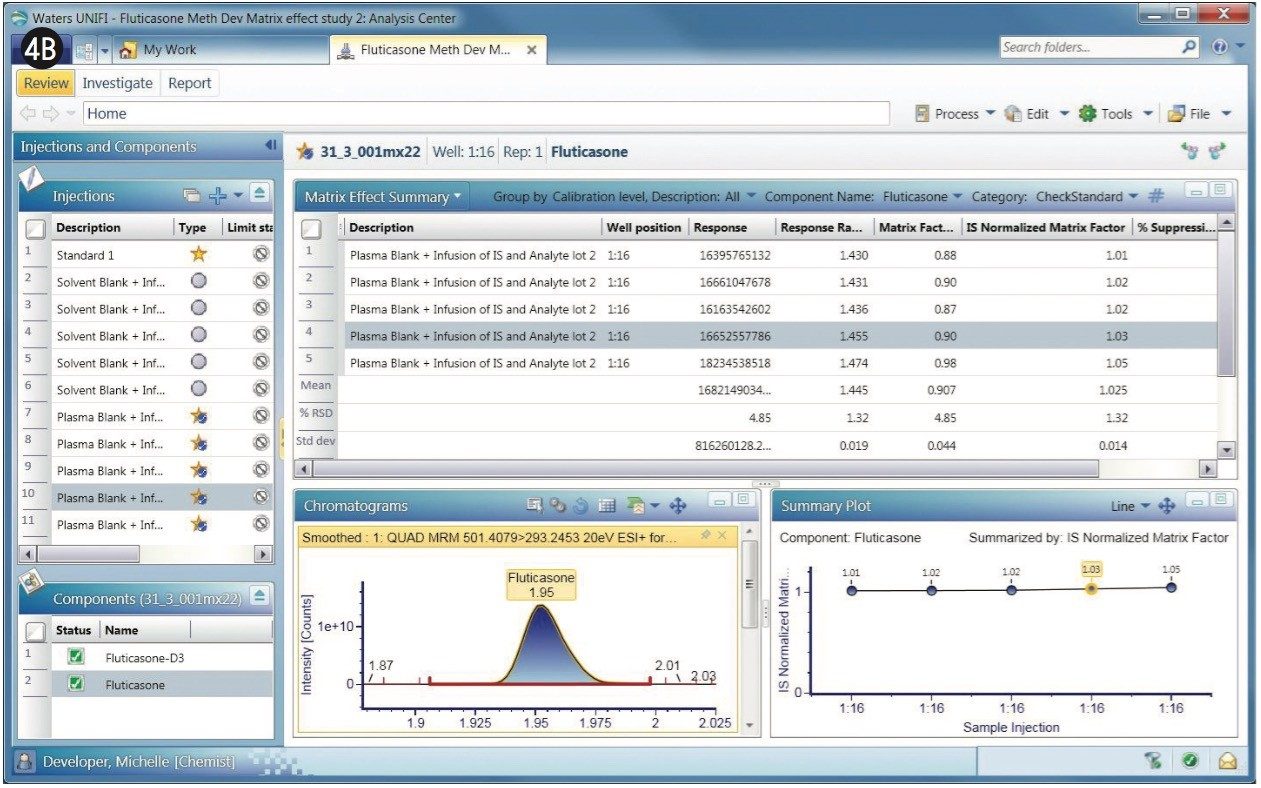
The matrix factor for the 2-min gradient for fluticasone propionate with its internal standard is shown in Figure 4A (left), which shows the MRM of fluticasone propionate with the matrix factors of several replicate determinations in the summary plot.
The matrix factor of 0.90 obtained for fluticasone propionate differed significantly with the 2-min gradient, compared to that of the 1-min gradient, where it was 0.35. This implies that the analyte has moved a region of less interference when using the longer 2-min gradient.
As illustrated, the Waters Regulated Bioanalysis System Solution featuring UNIFI software provides a simple, automated, and convenient approach to quantifying the matrix factor of an assay. Depending on chromatographic conditions, analytes can coelute with endogenous components in matrix and/or drug-derived metabolites. This coelution can lead to significant matrix effects and possible reduced assay robustness and accuracy.
This automated approach to the determination of the matrix factor eliminates the need to employ third-party software such as Microsoft Excel to determine the matrix factor. All processes, from system setup, to data acquisition, processing, and calculations are carried out within a single secure, compliant-ready, audit-traceable system solution.
720004004, June 2011