Retention Without Compromise: Waters™ XSelect™ HSS T3 OBD™ Preparative Column for Polar Compounds
Jo-Ann Jablonski, Kathy Lawrence
Waters Corporation, United States
Published on October 17, 2025
Abstract
Although reversed-phase HPLC is the most popular and widely used chromatographic mode for the analysis and isolation of compounds from sample mixtures, polar compounds present unique issues in reversed-phase separations. The challenge for the chromatographer who analyzes and isolates polar compounds revolves around getting sufficient retention on the non-polar stationary phase in a reversed-phase column. Waters developed a reversed-phase stationary phase designed for enhanced polar compound retention, XSelect HSS T3. Superior stability under low pH conditions and compatibility with 100% aqueous mobile phases make HSS T3 a prudent choice for polar compound purification and isolation.
Benefits
Waters XSelect HSS T3 OBD Preparative Columns:
- Provide increased retention for polar targets which simplifies isolation and purification strategies by eliminating laborious method development
- Are compatible with 100% aqueous mobile phases which mitigates the de-wetting of the stationary phase and subsequent target loss
- Are manufactured with high strength silica for mechanical stability resulting in long column lifetime
- Provide full scalability from UPLC™ to prep for predictable target collection using Waters' highly controlled OBD (Optimum Bed Density) column packing process, ensuring that preparative columns are of similar bed density to analytical columns of the same chemistry.1
Introduction
Although all compound isolations are potentially difficult to execute, especially when the targets are isolated from complex crude mixtures, a separation becomes particularly challenging when the target compound is not retained on the preparative column. Many chromatographers employ C18 columns for most of their target compound isolations and consider them to be universally applicable. These columns generally retain relatively nonpolar compounds and the target and impurities are eluted from the column using increasing percentages of organic solvent, usually acetonitrile or methanol.
Polar compounds, however, present a different challenge because they may not retain well on the nonpolar stationary phase, precluding purification. Sometimes polar compound retention can be improved by reducing the organic solvent concentration of the mobile phase for the initial conditions. However, high concentrations of water in the mobile phase can de-wet the nonpolar stationary phase, which may lead to a loss of retention and loss of target compound.2 Simply put, polar compounds are notoriously difficult to retain on traditional C18 columns. Even with method tweaks, chromatographers often battle poor retention, peak distortion, or outright target loss.
The XSelect HSS T3 phase was purpose-designed to solve this challenge, delivering strong, reliable retention for polar analytes, compatibility with 100% aqueous mobile phases, and scalability from UPLC to prep LC. The HSS T3 stationary phase, with its lower carbon content and ligand bonding density, is particularly suited for the analysis and purification of polar compounds. The high strength silica base particle of the HSS T3 phase provides mechanical stability for the demands of purification.
Experimental
Sample Description
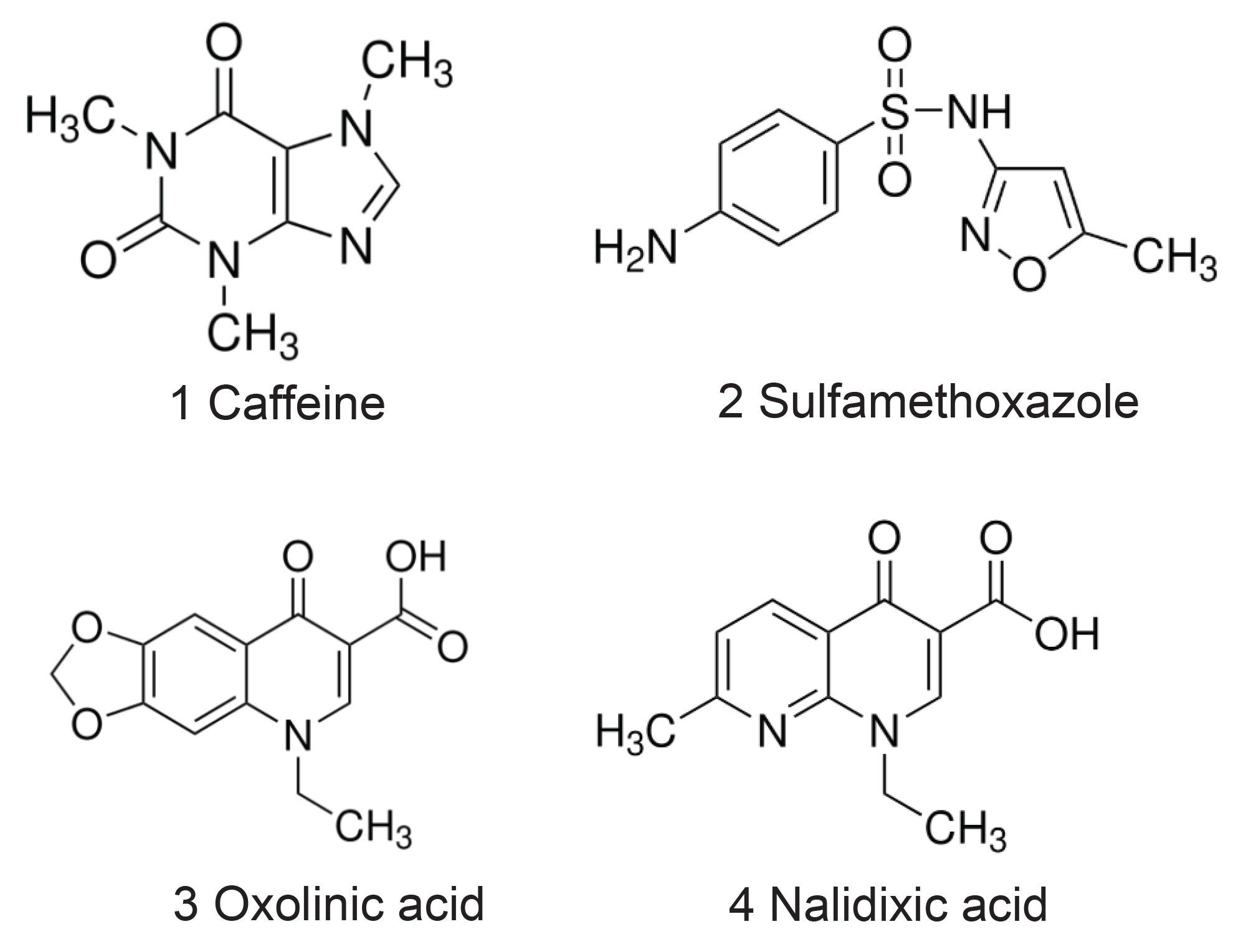
A mixture composed of 4 compounds of various polarities was prepared to a final total concentration of 35 mg/mL in dimethylsulfoxide (DMSO) and filtered using a 25 mm, 0.45 µm PTFE CR Acrodisc™ Syringe Filter (Cytiva). Figure 1 shows the structures of the compounds in the sample mixture.
LC Conditions
|
LC systems: |
Waters AutoPurification System ACQUITY™ UPLC H-Class System |
|
UV detection: |
AutoPurification System: 2998 Photodiode Array Detector ACQUITY H-Class System: TUV Detector Wavelength: 254 nm |
|
Columns: |
ACQUITY UPLC HSS T3, 1.8 µm Column, 2.1 x 50 mm, p/n: 186003538 XSelect HSS T3 OBD Preparative Column, 130 Å, 5 µm, 30 x 150 mm, p/n: 186005790 |
|
Column temperature: |
Ambient |
|
Sample temperature: |
Ambient |
|
Sample loop: |
5000 µL; stainless steel |
|
Injection volumes: |
As noted in figures |
|
Flow rates: |
Analytical 0.7 mL/min; Prep 48.6 mL/min |
|
Mobile phase A: |
0.1% Formic Acid in Water |
|
Mobile phase B: |
0.1% Formic Acid in Acetonitrile |
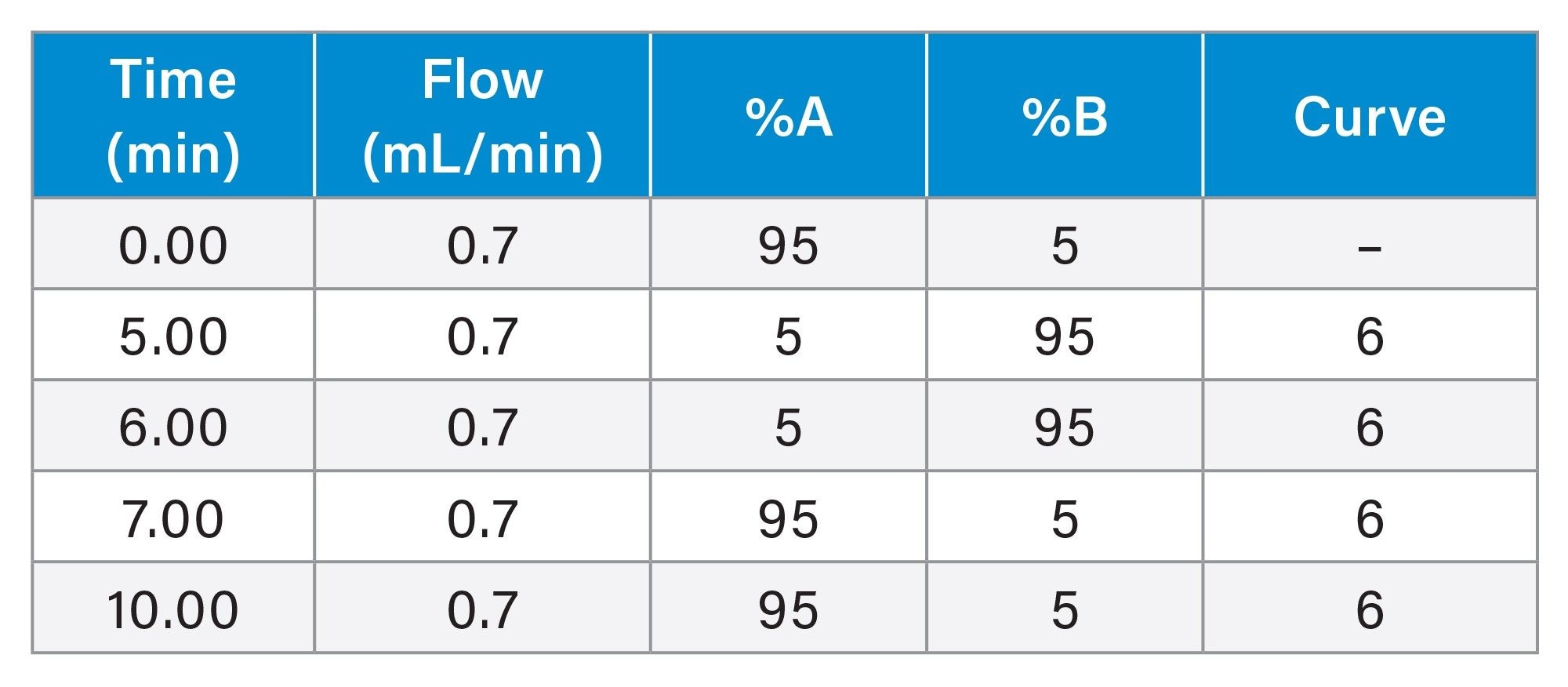
Gradient Table: Analytical Scouting Method
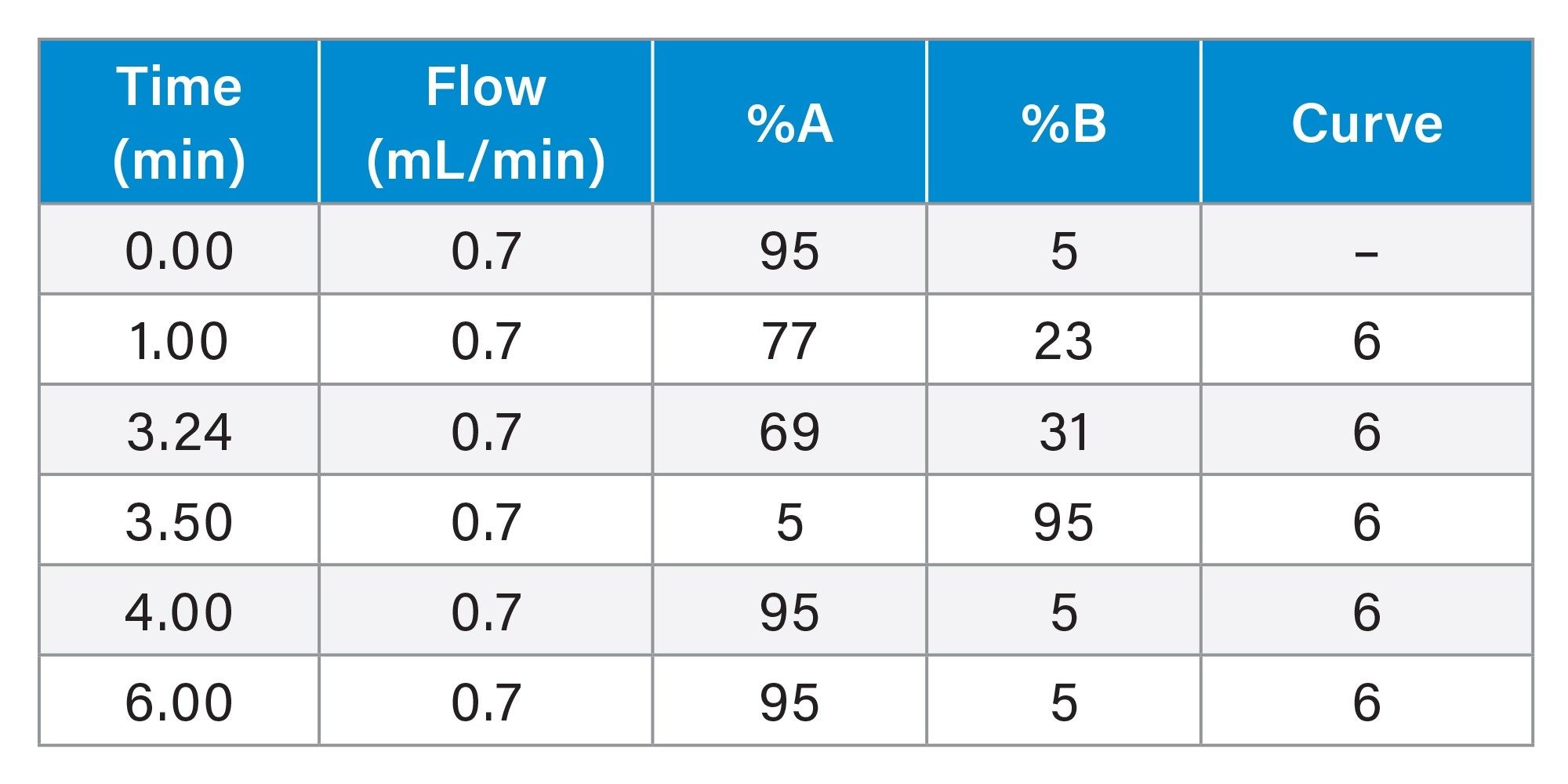
Gradient Table: Analytical Focused Gradient Method
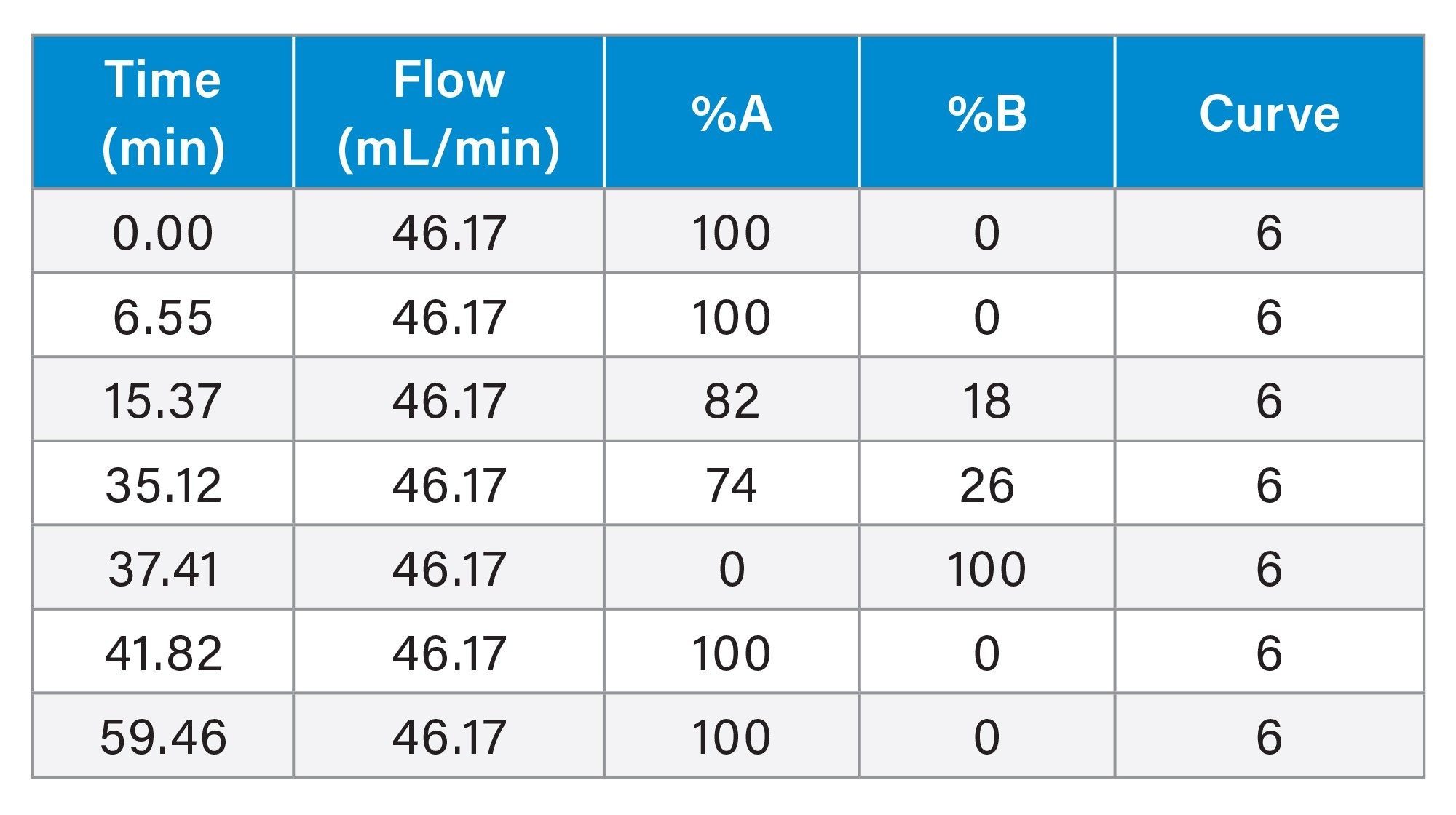
Gradient Table: Preparative Method
Data Management
|
Chromatography software: |
MassLynx™ version 4.2 Software |
|
Application manager: |
FractionLynx |
Results and Discussion
Waters XSelect HSS T3 Columns are manufactured with high strength silica particles that provide mechanical stability for the demands of purification. The HSS T3 stationary phase has a lower carbon content and bonding density (11% and 1.6 µmol/m2, respectively) compared to that of an HSS C18 stationary phase (15% and 3.2 µmol/m2).3 The Waters proprietary T3 trifunctional bonding and endcapping technology yields column packing materials with superior low-pH stability.4
Table 1 shows the column attributes, as well as the system and experimental parameters for the evaluation performed on the XSelect HSS T3 Column. Although these commonly reported properties describing a column packing are readily available, they do not exclusively provide an indication of retentivity, capacity, or efficiency. Additional separation parameters, such as sample diluent and solubility, LC method conditions, and the resolution between sample mixture components, influence the chromatographic outcomes achieved during analysis and purification.
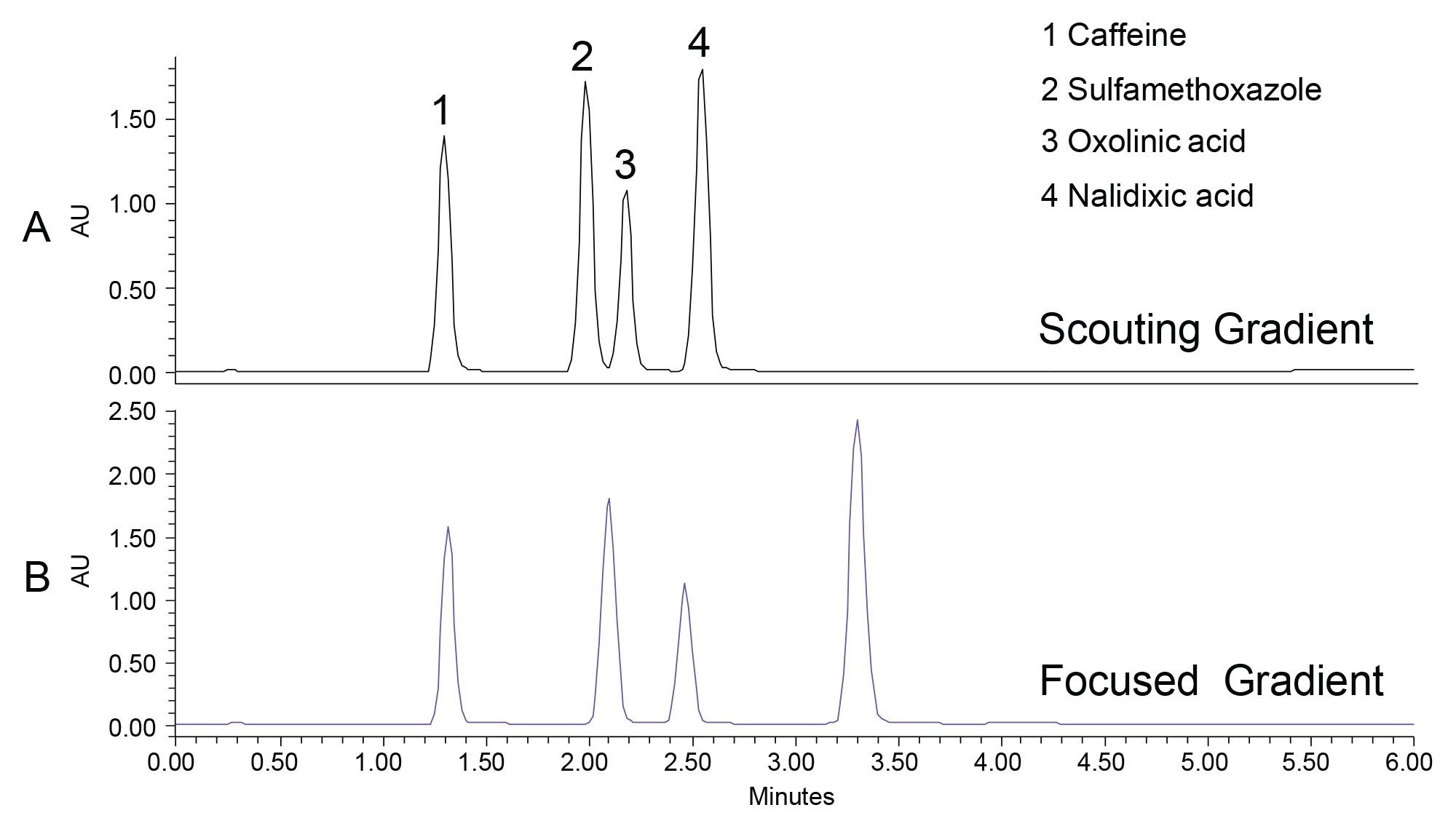
In this study, a 4-compound chemical mixture composed of the compounds shown in Figure 1 was analyzed using the ACQUITY UPLC HSS T3, 1.8 µm, 2.1 x 50 mm Column. Although sulfamethoxazole and oxolinic acid were baseline resolved from one another, the oxolinic acid eluted immediately after the sulfamethoxazole (Figure 2A).
In preparing for a subsequent prep LC separation, sulfamethoxazole was identified as the target compound for isolation. Focusing the gradient5 increased the resolution between sulfamethoxazole and oxolinic acid resulting in a method that was more suitable for increased sample load in purification (Figure 2B).
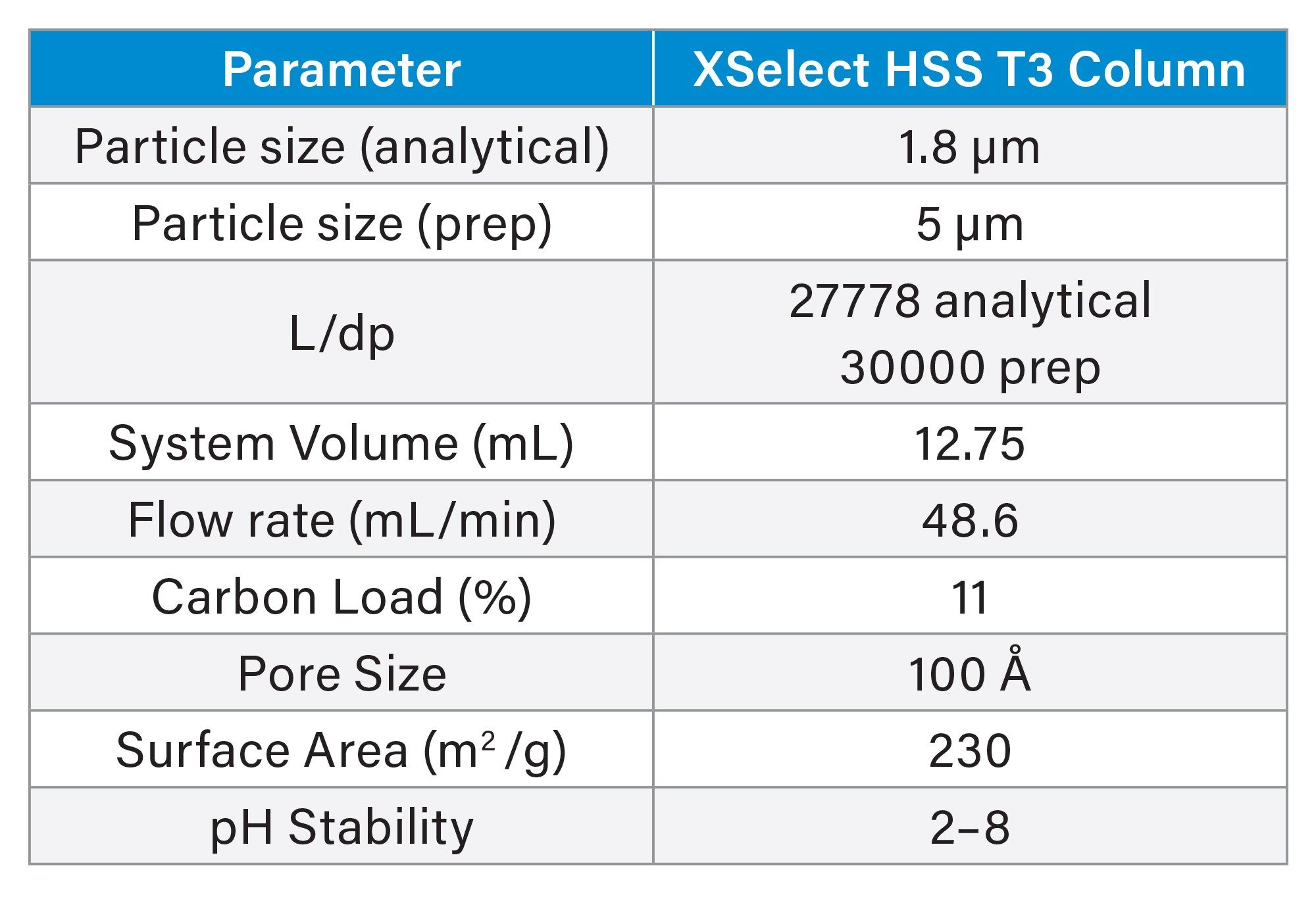
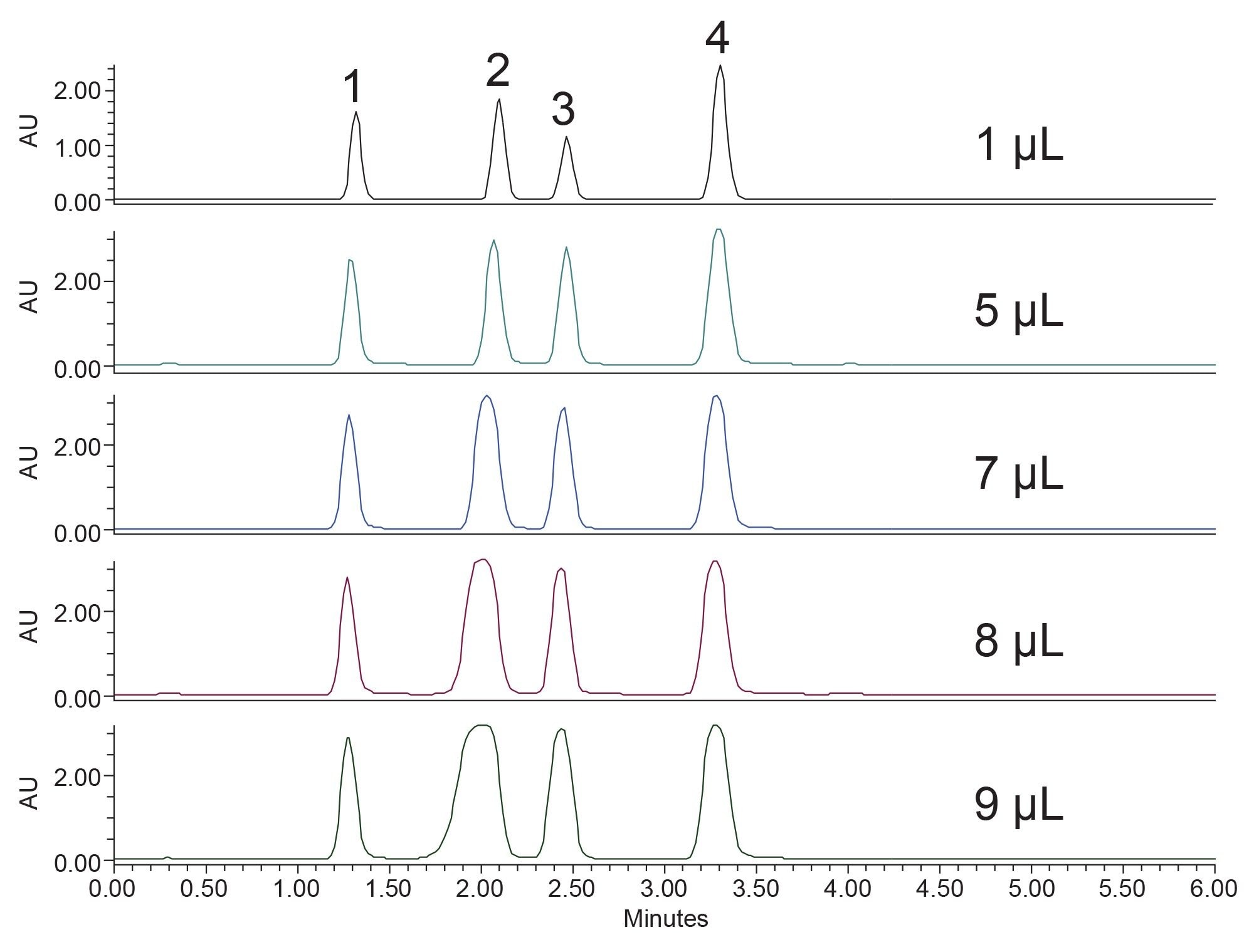
To maintain a separation when scaling from UPLC to prep LC, the ratio of the length of the column to the diameter of the particle, or L/dp, should be as close to the same as possible.6 Table 1 shows the L/dp for the analytical and prep columns used in this study, and, although the ratios are not identical, they are very close in value. The analytical focused gradient methods were scaled for prep7 on the 30 x 150 mm, 5 µm XSelect HSS T3 Column. A loading study on the analytical column using the focused gradient indicated that injecting 9 µL (0.3 mg) of the sample would still give adequate resolution between sulfamethoxazole and oxolinic acid (Figure 3). Geometric scaling to prep indicated that as much as 193 mg (5.5 mL) could be purified in each chromatographic run, however the injection volume was reduced to 2 mL (70 mg) to more appropriately align with the system configuration.
System volume is defined as the volume from the point of gradient formation to the head of the column and includes the sample loop. The prep system was configured with a 5 mL sample loop to accommodate the predicted large injection volumes. Accounting for the system volumes during scale-up helps to ensure chromatographic outcomes more closely resemble the analytical scouting run. The system volumes of the analytical and preparative systems were measured at 0.378 mL and 12.75 mL, respectively.8
1 = Caffeine, 2 = Sulfamethoxazole, 3 = Oxolinic acid, 4 = Nalidixic acid
As mentioned previously, the sample mixture was dissolved in a strong solvent (DMSO) and large injection volumes of strong solvents may distort chromatographic peaks. After injection, the strong solvent immediately starts moving down the column, carrying the sample with it. With large amounts of strong solvent sample diluent, the sample compounds will not adsorb onto the column until the strong solvent is sufficiently diluted for them to interact with the column stationary phase. By then, the sample bands are broadened, giving poor peak shapes and ultimately leading to impure fractions and potential sample loss. Because the large, 2 mL sample injection volume would potentially produce distorted chromatography, at-column dilution9 was employed. At-column dilution is an injection technique which allows the purification scientist to inject large volumes of strong solvents, increase sample load, and obtain improved peak shape for better fraction collection.
Figure 4 shows the preparative separation obtained on the XSelect HSS T3 OBD Preparative Column. To save time and solvent, the prep method was terminated shortly after the elution of nalidixic acid. The preparative column was also evaluated in the Waters column manufacturing facility. Table 2 shows the method conditions, while Table 3 shows the tabulated experimental results from three injections of the acetone/acenaphthene sample mixture. From this QC test mix evaluation, it is evident that the efficiency, pressure, and retention time reproducibility are excellent.
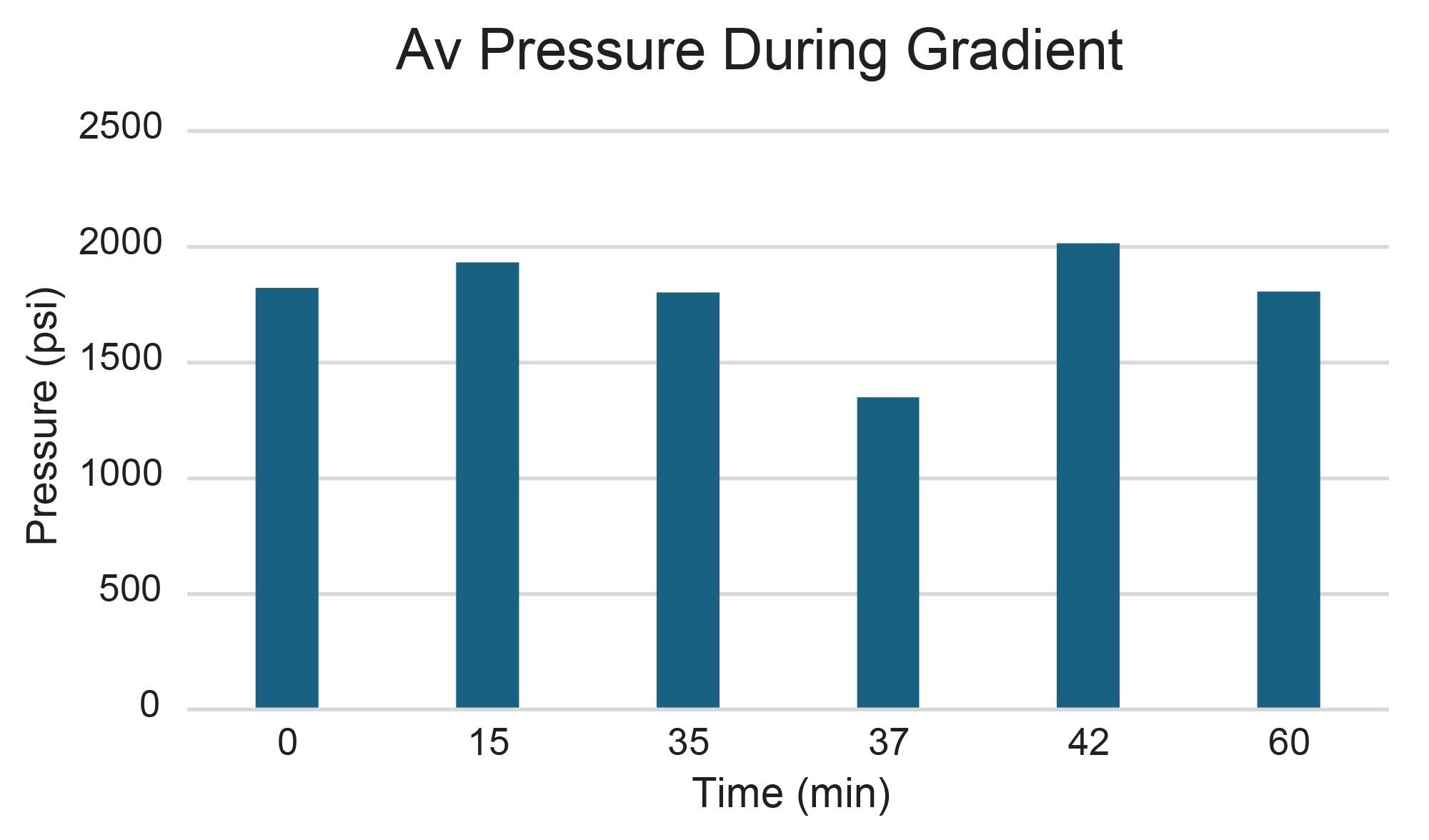
Total pressure during a chromatographic run is the sum of the system pressure and the column pressure. The system pressure includes all components of the flow path and is directly influenced by the inside diameter of the tubing connecting said components at the specified flow rate. Purification scientists are concerned about the pressure during a chromatographic separation because high pressure could be an indication of sample precipitation and the potential for sample loss. If pressure spikes occur, they must be well below the upper pressure limit of the prep LC instrumentation to avoid a system shutdown. Pressures below the maximum pressure limit on the prep LC system increase the likelihood of sample recovery if a sample does precipitate due to other factors, such as incomplete solubility in the mobile phase.
Pressure readings were noted over the course of the gradient separations performed on the 30 x 150 mm preparative column. These results are shown in Figure 5. At time points throughout the gradient, the pressure was well below the preparative LC instrumentation upper limit of 6000 psi.
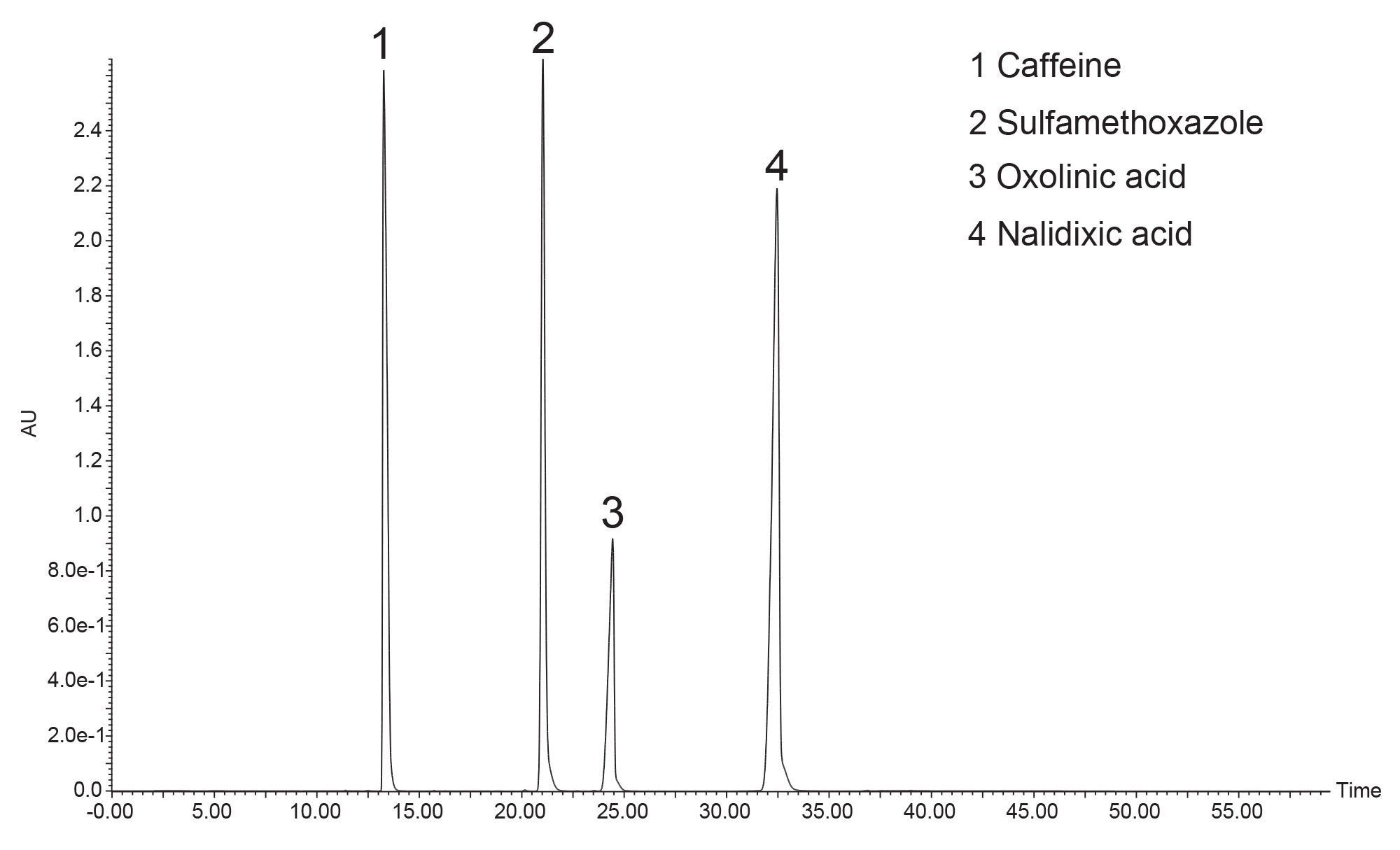
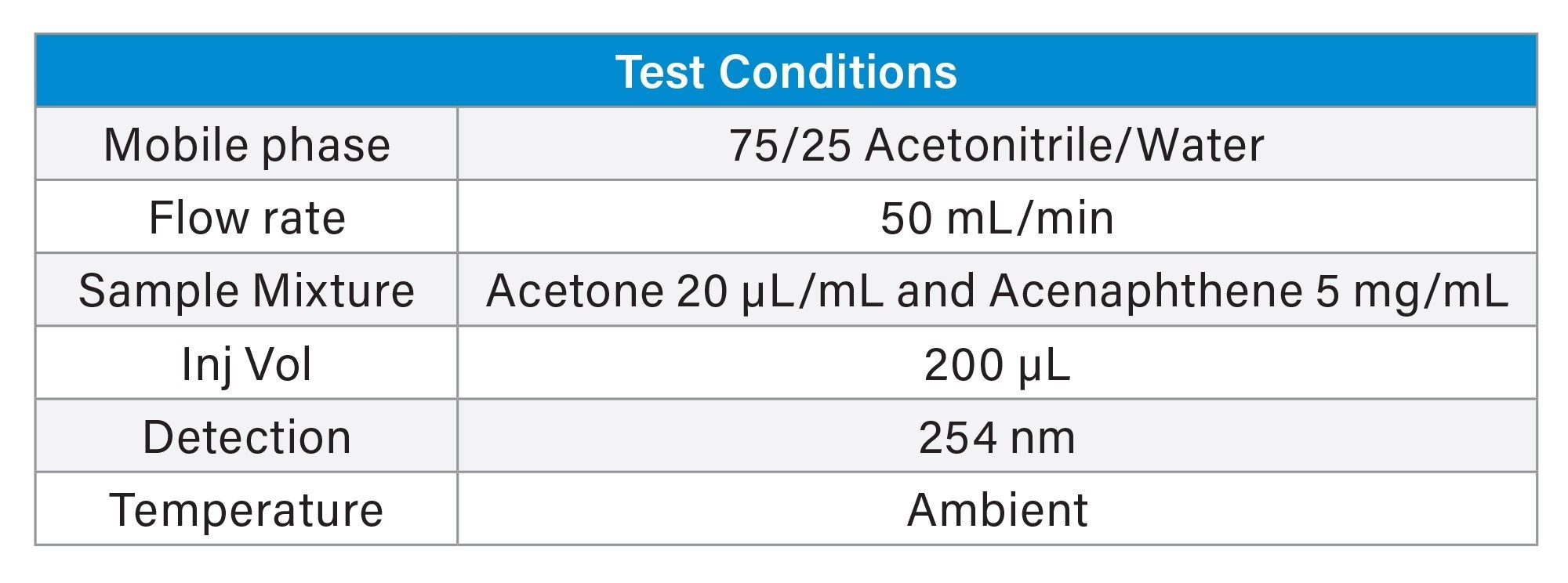
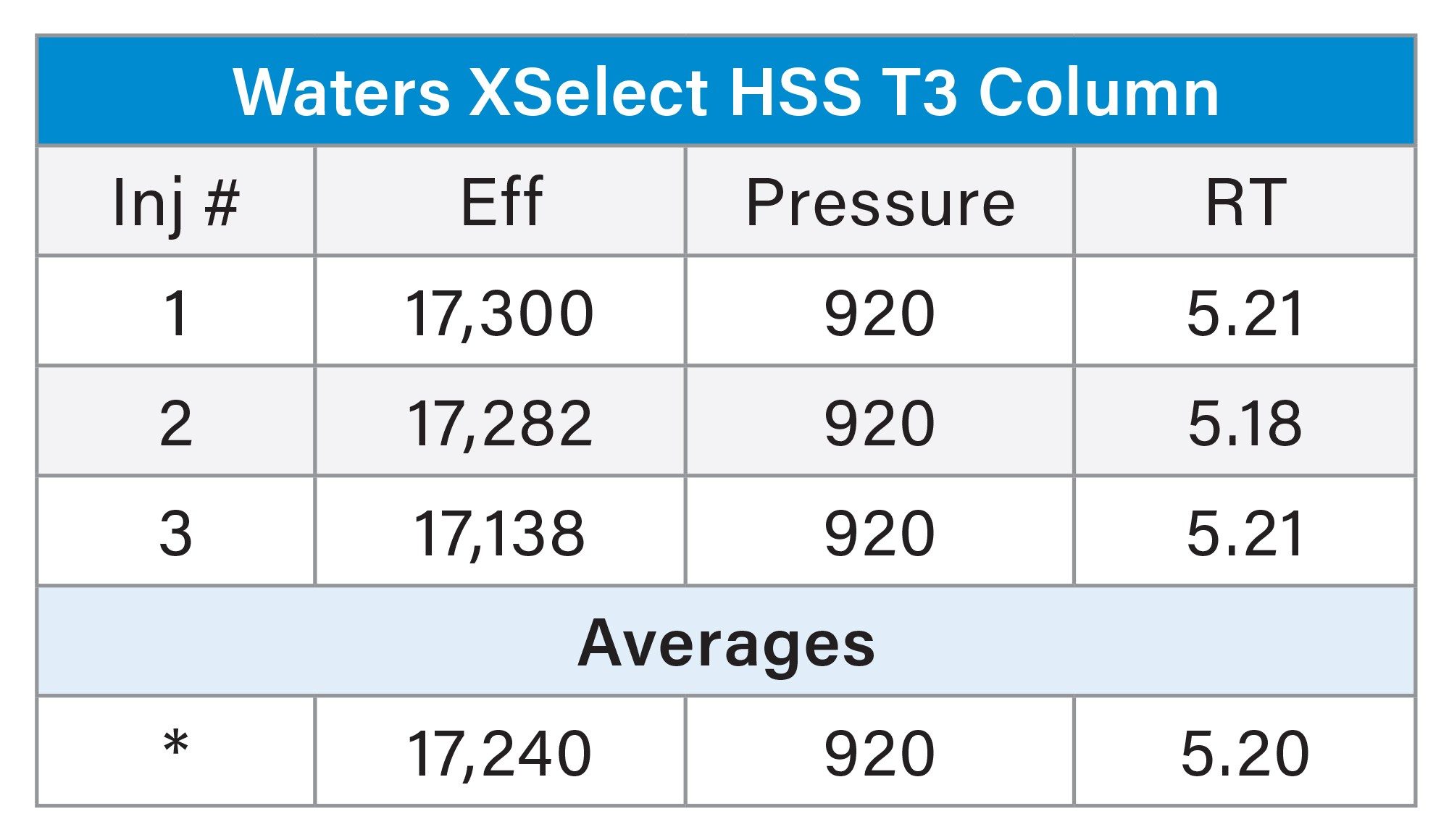
Sample mixture: Acetone (20 µL/mL) and Acenaphthene (5 mg/mL).
Conclusion
Isolating polar compounds from crude mixtures may also entail the fractionation of nonpolar entities from the same sample. Waters XSelect HSS T3 Columns provide a viable alternative for the isolation of both polar and nonpolar compounds in a single chromatographic run. The HSS T3 stationary phase packing in these columns provides a C18 bonded and endcapped phase bound to a high-strength silica substrate designed for exceptional polar compound retention. As such, these columns can be an ideal choice for chemists seeking a C18 column that is appropriate as a universal column, providing full scalability from UPLC/UHPLC to prep, high reproducibility and good retention of problematic polar compounds.
Scaling from UPLC to prep is easily realized when the rules of scaleup are employed.10 Both analytical and preparative XSelect HSS T3 Columns provided nearly identical chromatographic outcomes for a mixture of four polar compounds where experiments detailed the analysis, focusing, and preparative purification.
The 30 x 150 mm preparative column was evaluated in the Waters column manufacturing facility using established experimental procedures to assess efficiency, retention time, and pressure. Whether operating under QC test or gradient conditions, the XSelect HSS T3 Column operated at stable pressures well below the upper pressure limit for the prep LC system. The efficiency and retention time results for the XSelect HSS T3 Column were highly reproducible, valuable attributes when isolating polar compounds.
References
- Topics in Liquid Chromatography, Part 2: Optimum Bed Density [OBD™] Columns: Enabling Technology for Laboratory-Scale Isolation and Purification. Waters Corporation. White Paper. 720001939 EN 2012.
- Gritti F, Gilar M, Walter T, Wyndham K. “Retention loss of reversed-phase chromatographic columns using 100% aqueous mobile phases from fundamental insights to best practice”, J. Chromatrogr. A 1612 (2020), 460662, doi: 10.1016/j.chroma.2019.460662.
- OBD Preparative Columns Ordering Guide. Waters Corporation. 720008381. 2024.
- McDonald PD, McCabe D, Alden BA, Lawrence N, Walsh DP, Iraneta PC, Grumbach E, Xia F, Hong P. Topics in Liquid Chromatography Part 1. Designing a Reversed-Phase Column for Polar Compound Retention. Waters Corporation. White Paper. 720001889. 2007.
- Jablonski J, Wheat T, Diehl D. Developing Focused Gradients for Isolation and Purification. Waters Corporation. Application Note: 720002955. 2009.
- Jablonski J. 5 Rules of Scaling LC Purification: Rule 4. Leverage Identical Column Attributes and Similar L/dp. Waters Corporation. Article. 720008383. 2024.
- Diehl D, Xia F, Cavanaugh J, Fountain K, Jablonski J, Wheat T. Method Migration from UPLC Technology to Preparative HPLC. Waters Corporation. Application Note. 720002375. 2007.
- Bridging the Performance Gap from Analytical to Prep. Waters Corporation Wall Chart. 720002117. 2023.
- Using At-Column Dilution in Liquid Chromatography. Waters Corporation. White Paper. 720008462, 2024.
- Guidance for first-time purification success for your precious targets. Waters Corporation. Infographic. 720008209. 2024.
720009080, October 2025