Cracking the Challenge: Automated Extraction of PFAS in Backyard and Store-Bought Cage-Free Eggs Using LC-MS/MS and Dual-Phase GCB/WAX Cartridges
Margot Lee, Kari Organtini
Waters Corporation, United States
Published on July 31, 2025
Abstract
Many consumers enjoy eating eggs from backyard chickens and remain unaware of the environmental and dietary factors that influence egg safety. Those who consume eggs from backyard chickens often believe their eggs are healthier, yet they may be unknowingly exposing themselves to per- and polyfluoroalkyl substances (PFAS) contamination from sources like the environment, bedding, food, and drinking water. This study compares PFAS concentrations in store-bought cage-free eggs and cage-free eggs from backyard chickens, which may be exposed to a broader range of environmental factors and dietary variations due to free-range access and consuming kitchen scraps. A workflow for the analysis of whole egg (a dense, proteinaceous, and fatty matrix) is presented, utilizing automated sample preparation to reduce analyst involvement, minimize variability, and improve robustness when handling this challenging matrix. The automated sample extraction process takes less than 15 minutes per sample, and the automated solid-phase extraction (SPE) system can process up to 8 samples simultaneously in under 70 minutes. This method also uses dual-phase Waters™ Oasis™ GCB/WAX for PFAS Analysis Cartridges. The graphitized carbon black (GCB) and weak anion exchange (WAX) SPE cartridges clean up challenging samples to ensure precise and repeatable results across samples. Ultimately, this study aims to provide a clearer understanding of PFAS contamination in both commercially and locally sourced eggs, contributing insights into food safety through the creation of a consistent and efficient automated method for extracting PFAS from the challenging matrix of whole raw egg.
Benefits
- A streamlined and automated PFAS workflow for sample preparation of a complex matrix (eggs) with sensitive analysis on the Xevo™ TQ Absolute Mass Spectrometer to quantify at the sub-ng/g levels required to meet the guidance and regulations from the FDA and EU
- Use of dual-phase Oasis PFAS GCB/WAX Cartridges, combining 2 sorbents in one device, to streamline SPE, ensure cleanliness, and reduce false positives through QC-release testing for low residual PFAS
- The PFAS Solution Installation Kit reduces the risk of system and solvent contamination providing confidence in the accuracy of results
Introduction
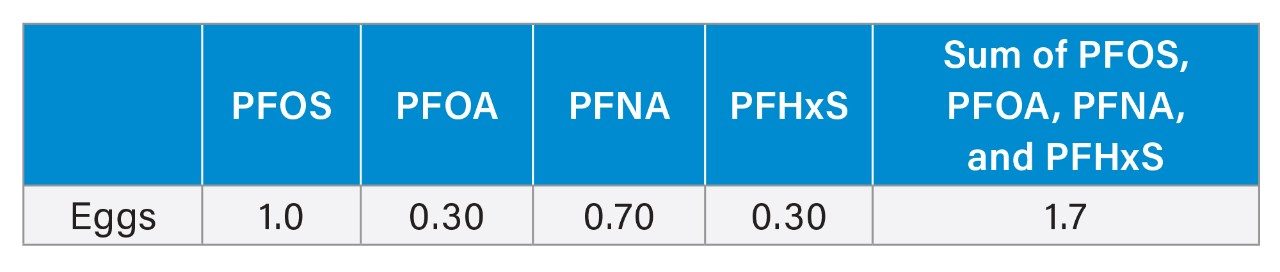
PFAS are known as potential contaminants in eggs, which are commonly consumed by humans. Chickens are exposed to PFAS through various routes, such as their diet, bedding, environment, and drinking water, with free-ranging chickens likely exposed to more variation in those factors.1, 3, 6 Unlike the European Union (EU), which has set maximum levels for 4 PFAS compounds (PFOS, PFOA, PFNA, and PFHxS)2, the United States currently has no federally established tolerable intake limits for PFAS in food. The European Food Safety Authority (EFSA) has also established a tolerable weekly intake (TWI) of 4.4 ng/kg body weight for the sum of these 4 compounds, which are known to contribute most significantly to PFAS levels in human serum.2 Although the Food and Drug Administration (FDA) has not set specific maximum intake levels for PFAS in food, it has issued requests for information on PFAS in food, shared testing results, and even issued import alerts. The collection and evaluation of the information could be used to establish future federal guidelines or regulations.4 There are currently 8 PFAS (PFOA, PFOS, PFNA, PFHxS, HFPO-DA (GenX), PFBS, PFBA, and PFHxA) for which there are toxicological reference values that are used to assess potential health concerns for levels found in food. These were evaluated by the FDA, though it deemed no present human health concern based on the levels that were found in the limited sampling of food.2 However, although the FDA conducted that testing in 2021, current advances in mass spectrometry now enable the detection of a broader range of PFAS at lower concentrations, allowing for a more comprehensive and accurate assessment of PFAS in food supplies.
Experimental
All standards were purchased from Wellington Laboratories. The following were used to prepare stock solutions:
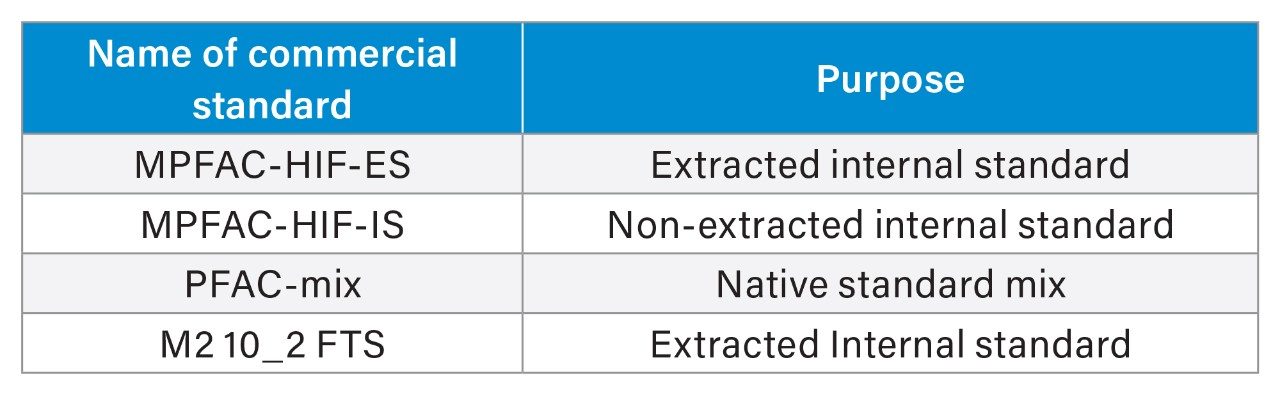
A native PFAS mix stock solution (500 ng/L of each analyte) was prepared in methanol and was used for serial dilutions. An extraction internal standard (EIS) solution was prepared in methanol and was used to spike egg samples prior to extraction. A mix of EIS and non-extracted internal standards (NIS) was prepared in a solution of ammonium hydroxide, water, acetic acid, and methanol, which served as diluent for the calibration curve. Lastly, the non-extracted internal standard (HIF-IS) was used to spike each eluted sample after extraction, prior to LC-MS/MS analysis.
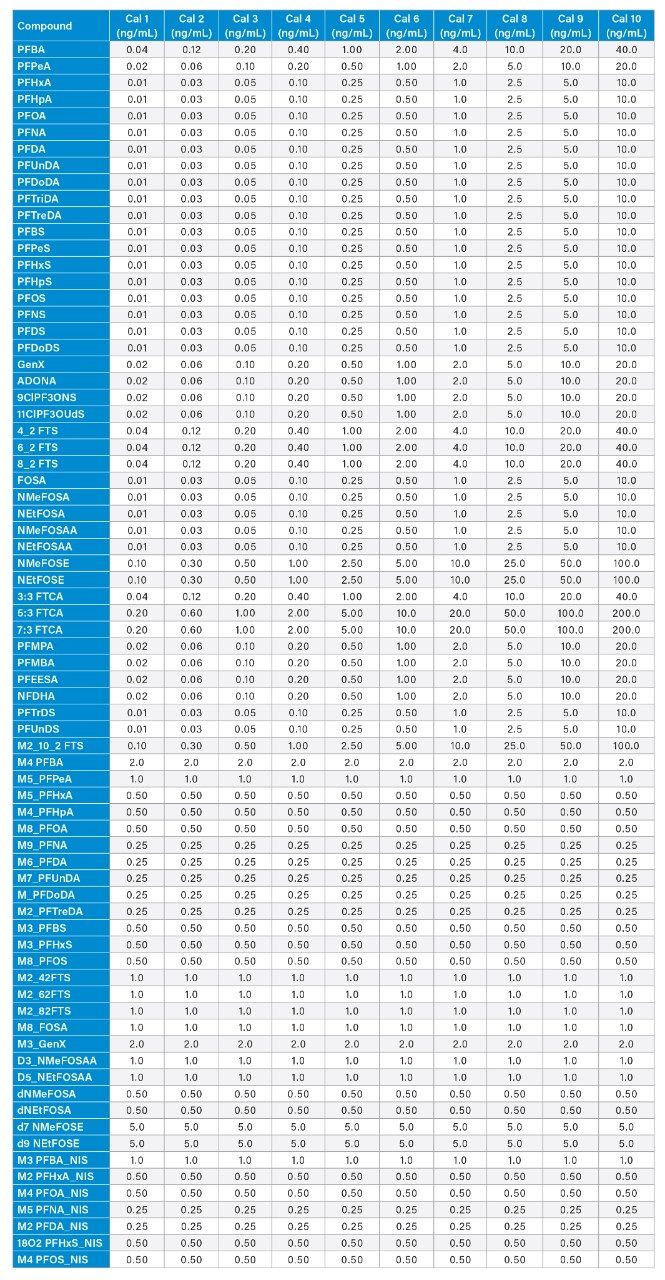
A 10-point solvent calibration curve in the range of 0.01 to 100 ng/mL was prepared and used for sample analysis. PFAS compound concentrations can be seen in Appendix Table 1.
Concentrations were reported in ng/g, with an EIS-spiked blank sample that was run each day subtracted from the egg samples prior to reporting values for an accurate representation of PFAS concentration (daily method blank correction). Because there is not an egg representative sample that could be used as a blank, the blank samples were 2.5 g of Q-matrix™ (CEM Corporation) that were spiked with EIS. Percent recoveries were calculated by subtracting the concentration in un-spiked eggs from that in native-spiked eggs, dividing the expected spike concentration, and multiplying 100%.
Sample Preparation
All native spiked egg samples and blanks were prepared in duplicate, and all other egg samples were prepared in triplicate. The LC-MS/MS acquisitions were also run in triplicate. Sample preparation was performed by extraction using an automated pressurized fluid extraction system (CEM EDGE PFAS®) followed by SPE on the PromoChrom Technologies SPE-03 Gen 4 Automated SPE System.
Add 2.5 g CEM eCleanUP Hydra and 2 g homogenous egg white and yolk mixture to assembled Q-Cup containing a Q-Disc PFAS stack
Spike samples respectively with EIS, Native PFAS, or leave un-spiked
Sample types: Native-spiked eggs, native-free eggs, method blanks, system blanksPlace Q-Cups in rack with 50 mL polypropylene conical collection tubes, and slide rack into place in the CEM EDGE PFAS™
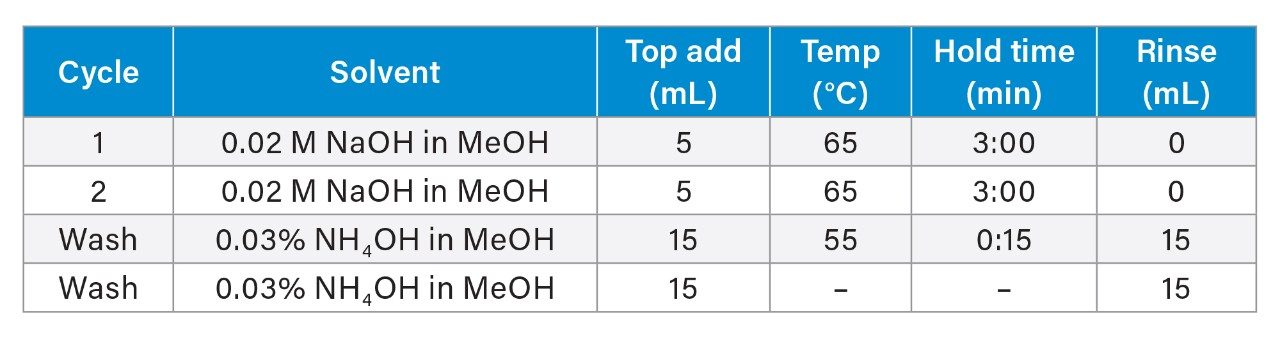
Start method for egg extraction: 1st cycle 5 minutes with 0.02 M NaOH and 2nd cycle with 5 minutes 0.02 M NaOHConcentrate collection to 2 mL (40 °C bath with nitrogen)
Reconstitute to 50 mL with LC/MS grade reagent water, vortex, check pH ≤6Load 50 mL sample on PromoChrom SPE-03 with Oasis™ GCB/WAX for PFAS Cartridges (p/n: 186011112) collecting in 15 mL polypropylene collection tubes
Run SPE method following method detailed in EPA 1633 with 5 mL eluteTo 5 mL eluted sample: add 25 µL acetic acid, spike with NIS, vortex
Aliquot 500 µL of sample in polypropylene vials and load on Waters Xevo™ TQ Absolute Mass Spectrometer for analysis
The dense, fatty, and viscous nature of the egg matrix makes extraction difficult. Low eCleanUP amounts (≤0.5 g) caused system errors and blockages, while higher loading improved dispersion, solvent flow, and sample stability. Ultimately, 2.5 g eCleanUP was chosen to ensure consistent performance.
Automated Method for PFAS in a 2 g Sample of Mixed Egg Yolk and White
LC Conditions
|
UPLC: |
ACQUITY™ UPLC™ I-Class PLUS System with PFAS Analysis Kit |
|
Mobile phase A: |
2 mM Ammonium Acetate in Water |
|
Mobile phase B: |
2 mM Ammonium Acetate in Acetonitrile |
|
Column(s): |
Analytical column: ACQUITY Premier BEH™ C18 Column 2.1 x 50 mm, 1.7 µm (p/n: 186009452) Isolator column: Atlantis™ Premier BEH C18 AX 2.1 x 50 mm, 5.0 µm (p/n: 186010926) |
|
Vials: |
700 µL Polypropylene Screw Cap Vials (p/n: 186005219) |
|
Column temperature: |
35 ˚C |
|
Sample temperature: |
8 ˚C |
|
Injection volume: |
2 µL |
|
Wash solvent: |
50:50 MeOH: H2O |
|
Purge solvent: |
10:90 MeOH: H2O |
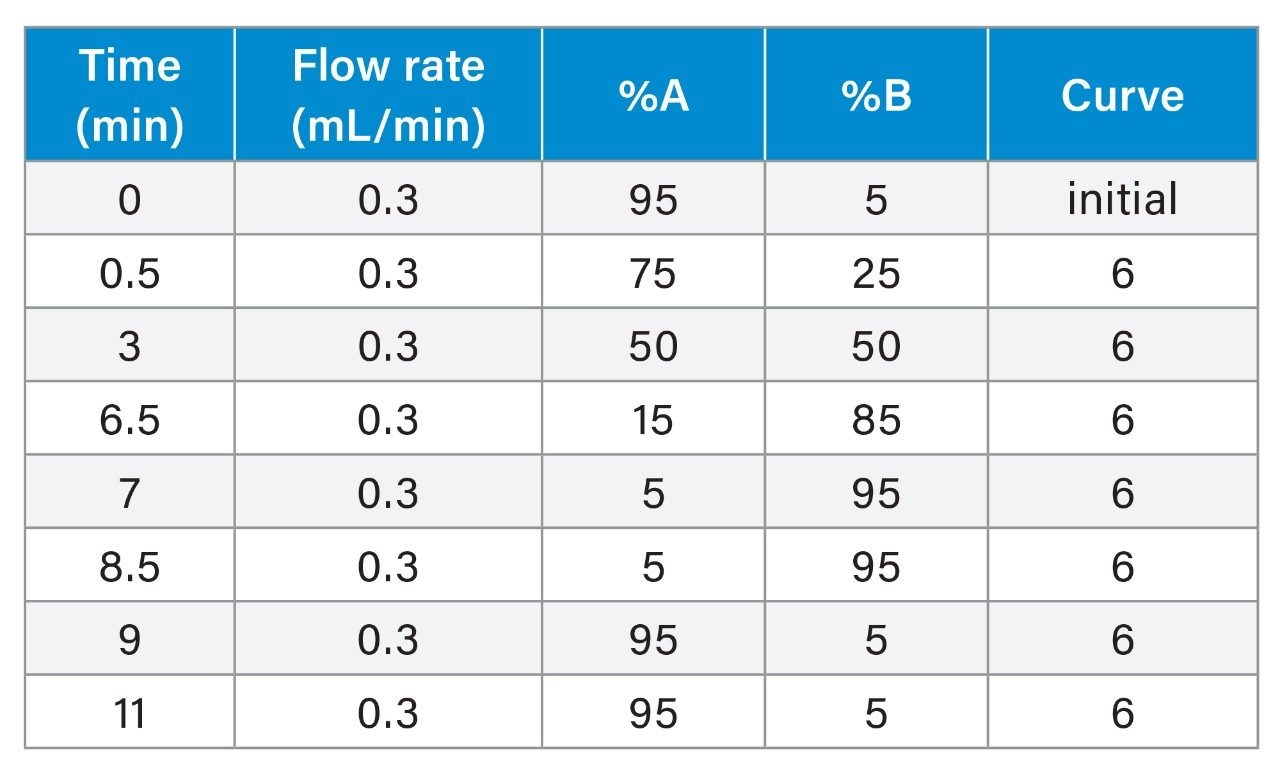
Gradient Table
MS Conditions
|
MS system: |
Xevo TQ Absolute Mass Spectrometer |
|
Ionization mode: |
ESI- |
|
Capillary voltage: |
0.5 kV |
|
Source temperature: |
100 °C |
|
Desolvation temperature: |
350 °C |
|
Desolvation flow: |
900 L/hr |
|
Cone flow: |
150 L/hr |
|
MRM transitions: |
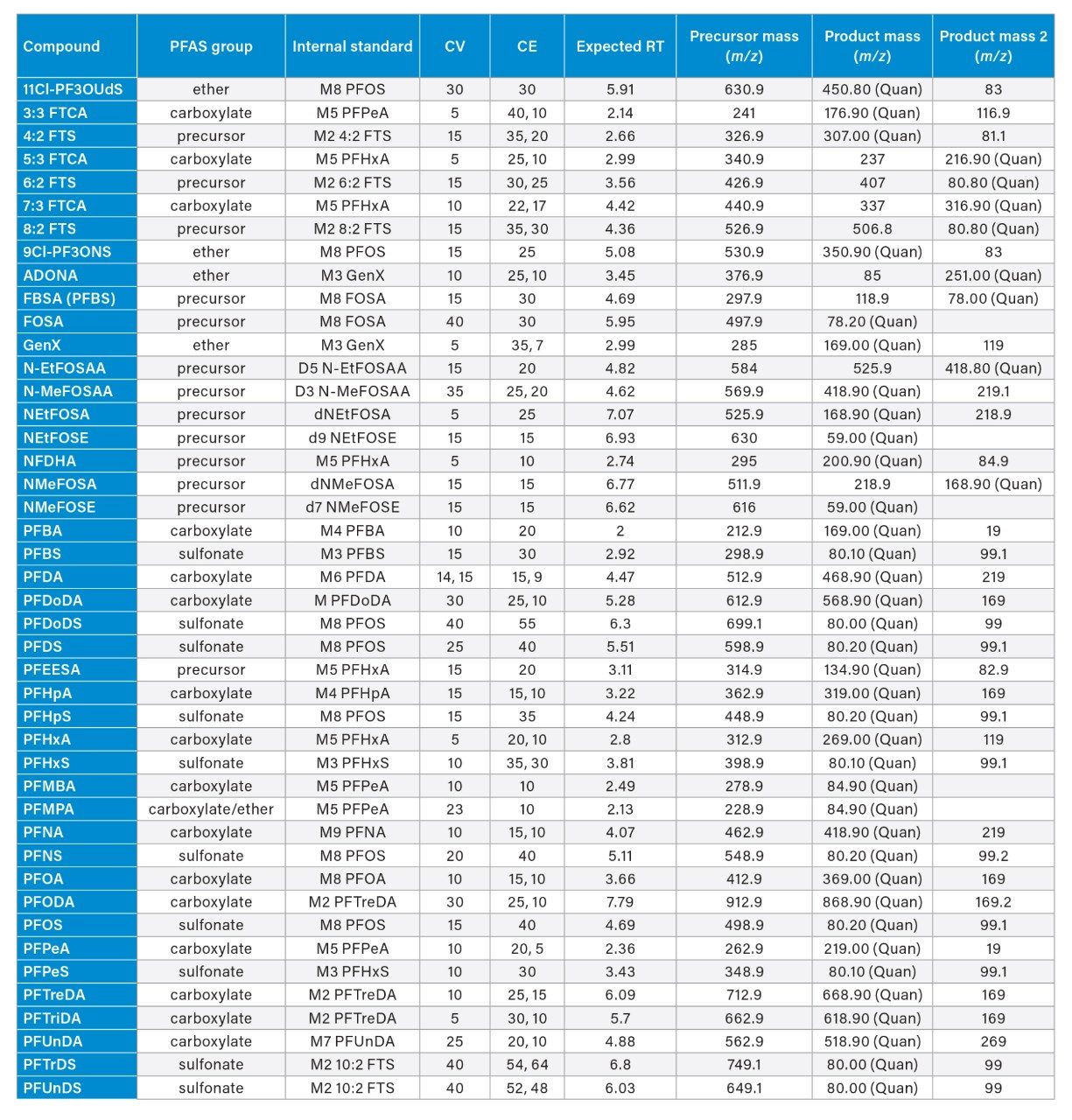
See appendix Table 2 |
Data Management
|
Software: |
waters_connect™ for Quantitation |
Results and Discussion
Egg samples were analyzed as described from 2 different flocks of backyard chickens located in 2 different Massachusetts towns, as well as from organic, cage-free eggs purchased from a local grocery store, which came from a farm in New York.
Most compounds showed acceptable recoveries within the 70–130% range, as seen in Table 4, demonstrating the method’s broad application across a diverse range of PFAS analytes. The 4 compounds of regulatory focus: PFOS, PFNA, PFOA, and PFHxS all fell within this range, supporting the robustness of the method for compounds under regulatory control. Notably, the complex whole egg matrix did not significantly compromise the recoveries for most analytes, underscoring the effectiveness of the automated extraction and SPE workflow in handling challenging food matrices.
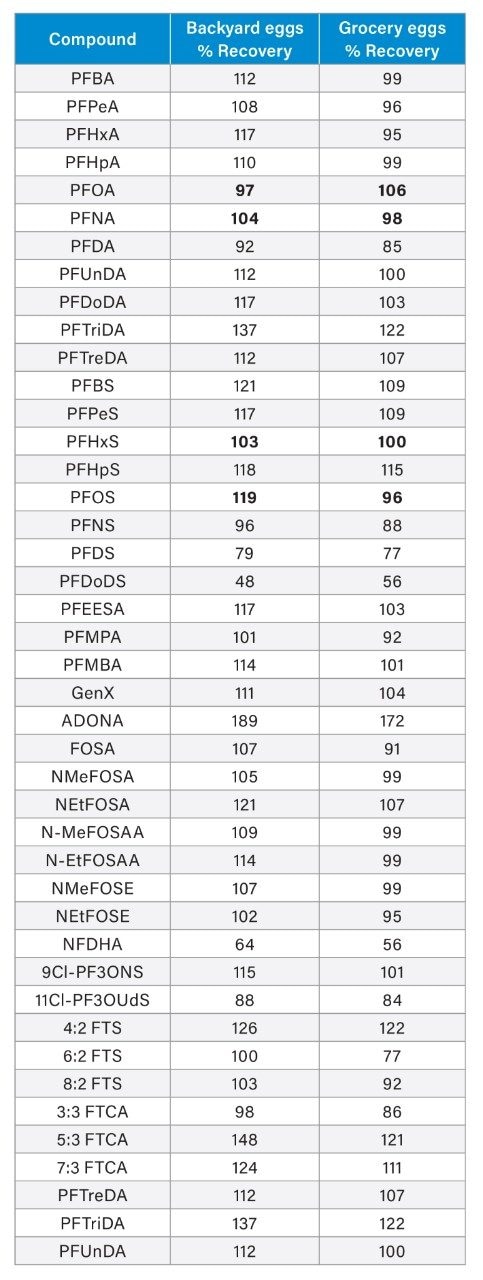
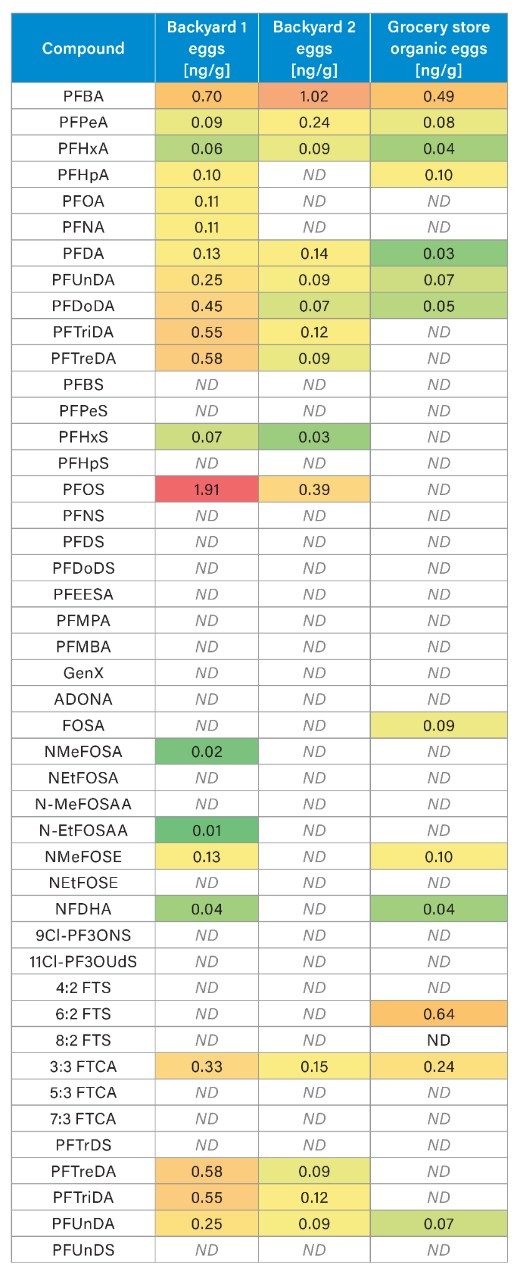
The heat map found in Table 5 uses color intensity to indicate PFAS concentration levels, with warmer colors representing higher values in ng/g, and ND indicating compounds not detected after blank correction. Of the 45 compounds analyzed, 24 were detected in at least one sample. PFBA, PFPeA, and PFOS were among the most abundant compounds, with PFOS reaching levels that approached or exceeded EU regulatory thresholds in some backyard eggs. One backyard was the site of a historical house and fuel fire incident where aqueous film-forming foam (AFFF), a documented source of PFOS contamination,7 was likely deployed. This legacy contamination may account for the elevated PFOS concentrations observed in the eggs from chickens raised at that location. In general, backyard eggs contained a greater number of PFAS at higher concentrations than store-bought eggs, likely due to increased exposure through roaming, diet, water, bedding, etc. Precursors like FOSA and FTS were only detected in grocery store eggs, suggesting legacy contamination from older food or packaging sources. Other precursors such as NMeFOSE and emerging PFAS like ADONA and GenX were not widely detected. Backyard eggs also exhibited greater variability in PFAS concentrations, further underscoring the influence of environmental factors on PFAS exposure in non-commercial settings.
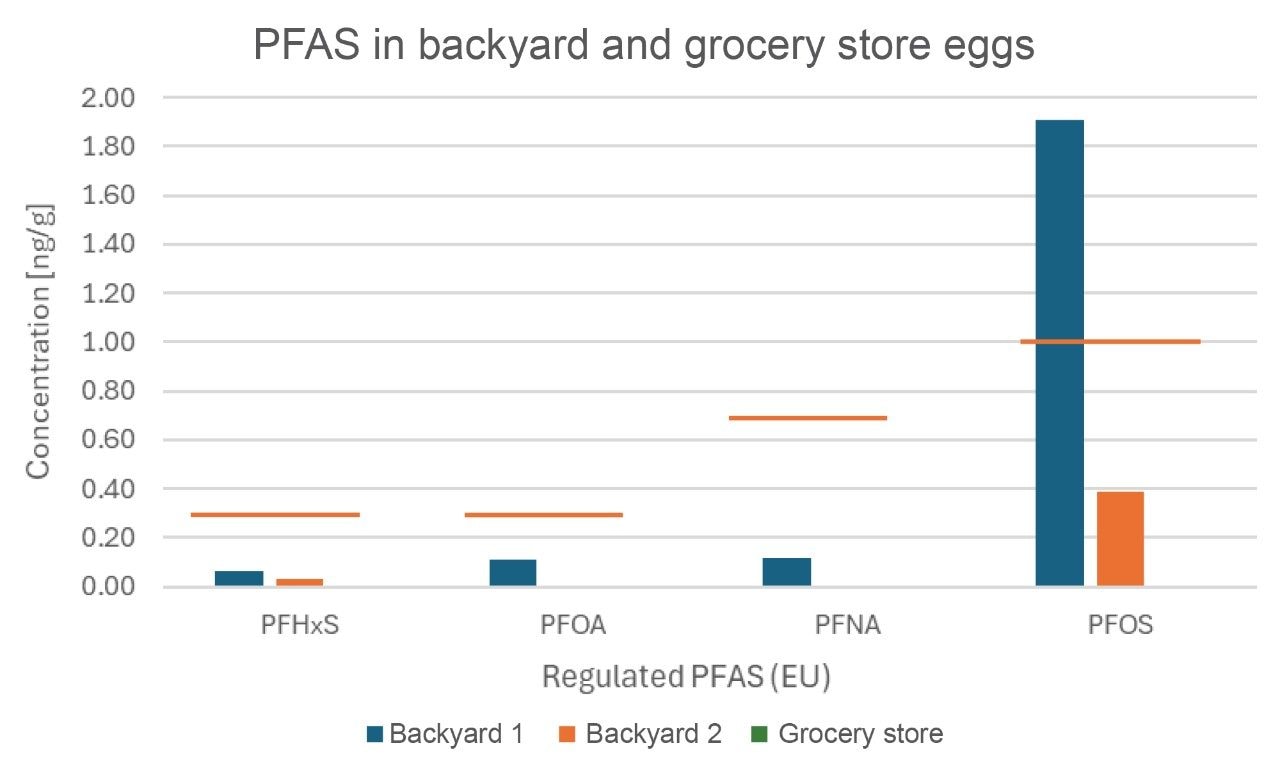
The figures below demonstrate the presence of the 4 compounds regulated by the EU and the 8 compounds that have been evaluated in food by the FDA in the backyard and grocery store eggs evaluated. Of the 4 PFAS regulated by the EU, grocery store eggs had no-detect levels, backyard 1 had detectable levels of all 4, with PFOS above the regulatory limit, and backyard 2 had only PFHxS and PFOS detected, both below the regulatory limit.
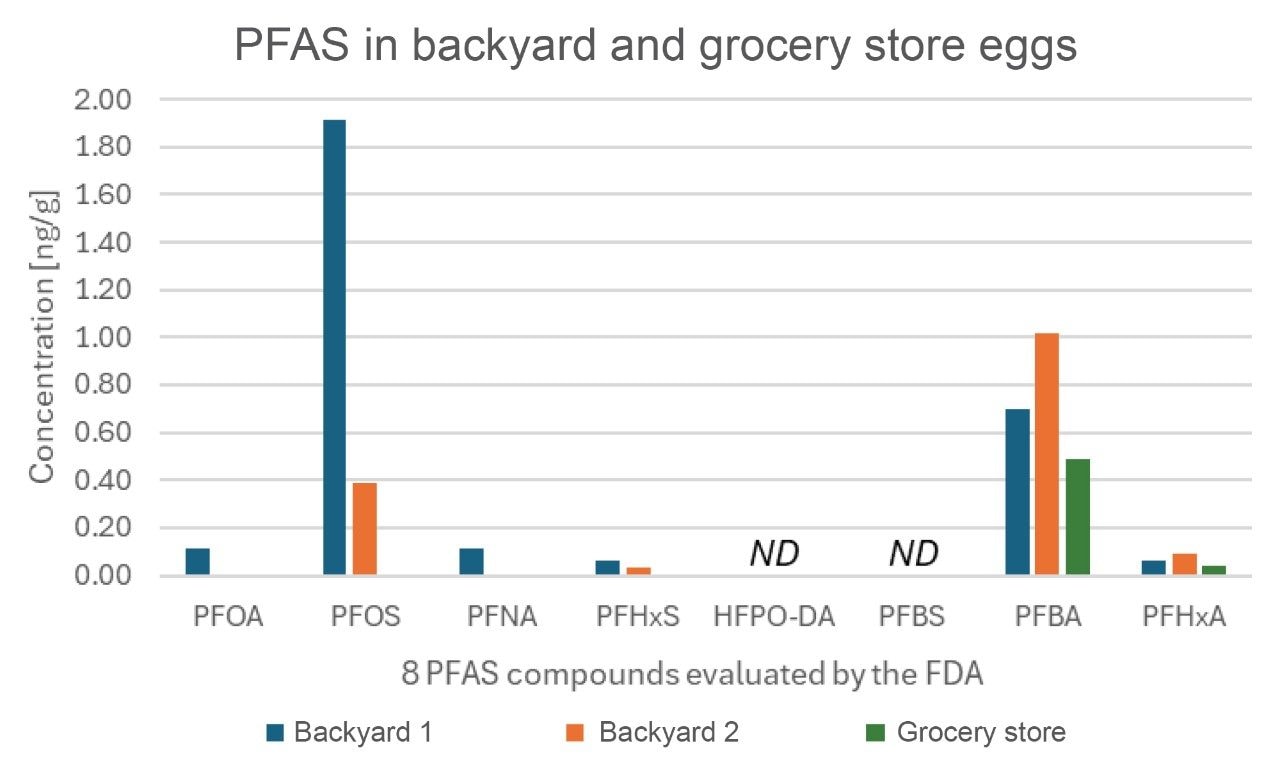
The FDA has evaluated the four PFAS regulated by the EU, in addition to HFPO-DA (GenX), PFBS, PFBA, and PFHxS. HFPO-DA and PFBS were not detected in the grocery store or backyard eggs. While the FDA does not have regulatory limits, it was noted all 3 sources contained PFBA and PFHxA, with backyard 2 having higher levels of both compounds than backyard 1 or the grocery store eggs.
Conclusion
The CEM EDGE PFAS and PromoChrom SPE-03 Systems, combined with the dual phase Oasis GCB/WAX for PFAS Cartridges, enabled efficient and reproducible extraction of PFAS from the challenging matrix of whole egg. Automation of the sample preparation and SPE steps delivered consistency across replicates and reduced overall method time. The workflow enabled the evaluation of PFAS compounds actively regulated by the EU. This method demonstrates that even difficult food matrices like whole egg can be prepared easily and reliably for PFAS analysis, supporting broader applications in food testing. Analysis revealed that PFOS and other PFAS were consistently higher in backyard chicken eggs compared to grocery store eggs, likely due to greater and differing environmental exposure from increased roaming space and dietary variation. PFAS precursors like FOSA were higher in grocery store eggs, likely due to potential legacy exposure. These findings highlight both the robustness of the automated method and the importance of monitoring PFAS contamination in non-commercial food sources. As awareness and monitoring of PFAS in food grows, utilization of this method offers a robust, high-confidence solution with limited user interaction — ideal for researchers and laboratories seeking to expand testing capabilities that not only meet but outperform regulated methods, while also future-proofing workflows for emerging PFAS compounds.
References
- Fernandes, Alwyn R., et al. "The Transfer of Environmental Contaminants (Brominated and Chlorinated Dioxins and Biphenyls, PBDEs, HBCDDs, PCNs and PFAS) from Recycled Materials Used for Bedding to the Eggs and Tissues of Chickens." Science of The Total Environment, vol. 892, 2023, p. 164441. Elsevier, https://doi.org/10.1016/j.scitotenv.2023.164441 Accessed on 02 June 2025.
- Official Journal of the European Union. 8.12.2022. Commission Regulation (EU) 2022/2388 of 7 December 2022 amending Regulations (EC) No 1881/2006 as regards to maximum levels of perfluoroalkyl substances in certain foodstuffs (section 10) accessed on 23 May 2025.
- Lasters, Robin, et al. “Home-Produced Eggs: An Important Human Exposure Pathway of Perfluoroalkylated Substances (PFAS).” Chemosphere, vol. 308, part 1, 2022, p. 136283. Elsevier, https://doi.org/10.1016/j.chemosphere.2022.136283. accessed on 23 May 2025.
- U.S. Food and Drug Administration. "Analytical Results of Testing Food for PFAS from Environmental Contamination." FDA, April 2025, accessed on 23 May 2025. https://www.fda.gov/food/environmental-contaminants-food/analytical-results-testing-food-pfas-environmental-contamination.
- U.S. Food and Drug Administration. "FDA Announcements on PFAS and Other U.S. Government Information." FDA, 18 Feb. 2025, www.fda.gov/food/process-contaminants-food/fda-announcements-pfas-and-other-us-government-information. Accessed on 27 May 2025.
- Zhang, Y., Wang, L., Li, J., Chen, H., and Liu, Y. "Occurrence and Risk Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Agricultural Soils and Crops: A Case Study in Eastern China." Chemosphere, vol. 350, 2024, p. 139123. Elsevier, https://doi.org/10.1016/j.chemosphere.2023.139123.
- Reinikainen, Jussi, Noora Perkola, Lauri Äystö, and Jaana Sorvari. "The occurrence, distribution, and risks of PFAS at AFFF-impacted sites in Finland." Science of The Total Environment, vol. 829, 2022, 154572. Elsevier, https://doi.org/10.1016/j.scitotenv.2022.154572.
Appendix
720008911, July 2025