For in vitro diagnostic use. Not available in all countries.
The Waters MassTrak Vitamin D Solution is designed for the quantitative determination of serum or plasma 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2), which in combination provide the total 25-hydroxyvitamin D concentration as an aid in the assessment of vitamin D sufficiency.
Assessment of vitamin D status is routinely performed to assess body stores of vitamin D and as an aid to diagnose; vitamin D deficiency or intoxication, intestinal mal-absorption, and to monitor adherence to therapy, and the therapeutic response in patients during treatment for vitamin D related disorders.
Vitamin D3 (cholecalciferol) is produced in the skin when the UV-B portion of sunlight converts 7-dehydrocholesterol to previtamin D3, which undergoes thermal isomerization to form vitamin D3. Latitude, season, aging, sunscreen use, clothing, and skin pigmentation influence production of vitamin D3 in the skin. Vitamin D2 (ergocalciferol) is produced through UV irradiation of ergosterol, which is found naturally in certain plankton, yeasts, and fungi and is used as a vitamin D dietary supplement in many countries1. Both vitamin D2 and vitamin D3 are metabolized, primarily in the liver, to 25-hydroxyvitamin D (25OHD). This accepted indicator of vitamin D status (Total 25OHD, which is the sum of 25OHD2 and 25OHD3) which has been a challenge to measure accurately because the antibodies used in many immunoassays do not have 100% co-specificity for both 25OHD2 and 25OHD3.
The Waters MassTrak Vitamin D Solution is designed for the quantitative determination of serum or plasma 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2), which in combination provide the total 25-hydroxyvitamin D concentration as an aid in the assessment of vitamin D sufficiency.
The MassTrak Vitamin D Kit, a component of the MassTrak Vitamin D Solution is validated for use with the Waters ACQUITY UPLC I-Class/Xevo TQD IVD System and the sample preparation has been validated using the Tecan Freedom 100/4 EVO Offline Automated Liquid Handling System.

|
System: |
ACQUITY UPLC I-Class with FTN |
|
Needle: |
30 μL |
|
Column: |
MassTrak Vitamin D BEH Phenyl 130 Å, 1.7 μm, and 2.1 mm x 50 mm IVD (p/n: 186008647IVD) |
|
Pre-Column filter: |
Column In-Line Filter Kit |
|
Mobile phase A: |
Aqueous 2 mM ammonium acetate with 0.1% formic acid |
|
Mobile phase B: |
Methanol with 2 mM ammonium acetate with 0.1% formic acid |
|
Column temp: |
35 °C |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.45 mL/min |
|
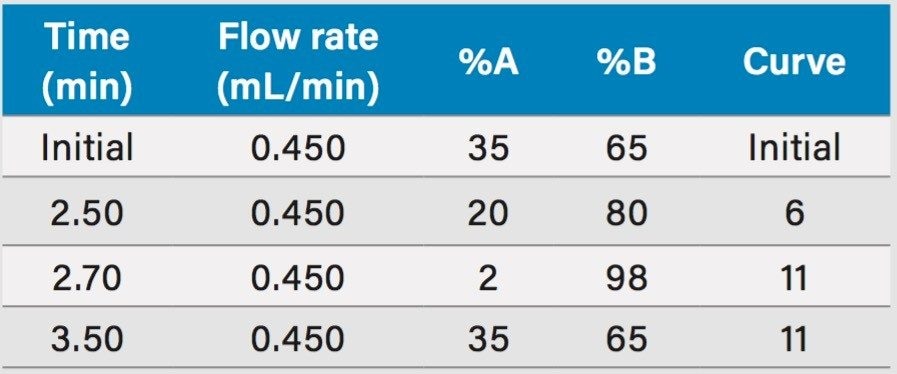
Gradient: |
See Table 1 |
|
Run time: |
4.2 minutes |
MassLynx v4.1 SCN 918IVD (p/n: 667005037IVD) with TargetLynx IVD v4.1 Application Manager (p/n: 667002671IVD)
Tecan File Converter Software v2.0 (p/n: 667004831)
|
System: |
Xevo TQD |
|
Resolution: |
MS1 (0.7 FWHM) MS2 (0.85 FWHM) |
|
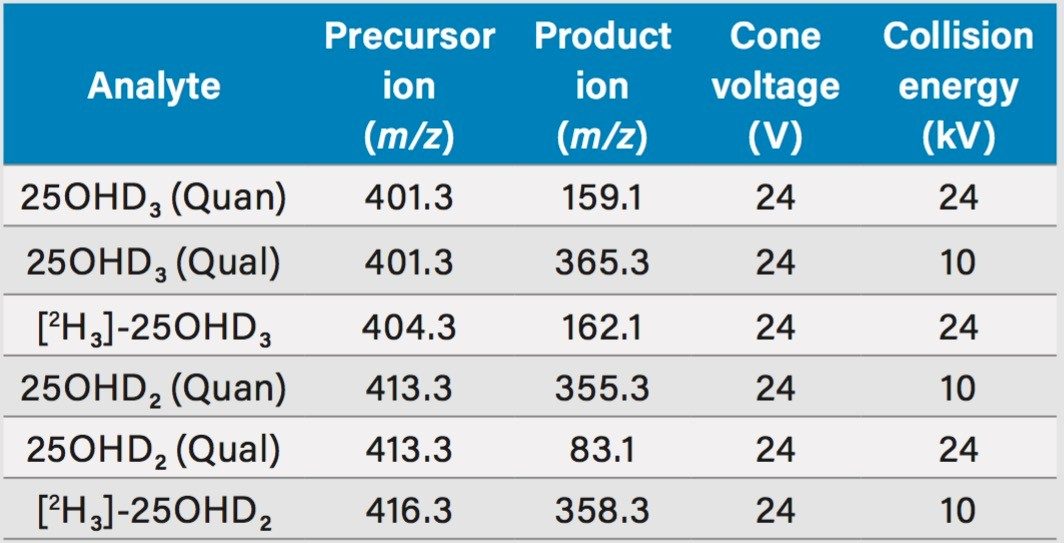
Acquisition mode: |
Multiple Reaction Monitoring (MRM) (see Table 2 for details) |
|
Polarity: |
ESI positive |
|
Capillary: |
0.80 kV |
|
Source temp.: |
120 °C |
|
Desolvation temp.: |
400 °C |
Refer to the Directions for Use (p/n: 715004830IVD) and the System Operator’s Guide (p/n: 715004831IVD)
The sample preparation process is automated for the MassTrak Vitamin D Solution and is performed using the Tecan Freedom EVO 100/4 Offline Automated Liquid Handling System.* The sample preparation consists of a series of steps that selectively extract 25OHD2 and 25OHD3 from the serum or plasma sample matrix ready for quantitative analysis by LC-MS/MS in less than two hours for 96 samples. A Tecan File Converter program is run on the Tecan workstation PC during sample preparation to collect the sample sequence information from the sample barcodes, and this is used to create an accurate sample list in the MassLynx Software.
A fixed quantity of internal standard solution is added to 150 μL of each test sample (calibrator, quality control, and patient sample). This is followed by the addition of zinc sulphate solution, which aids the release of the 25OHD from vitamin D-binding protein and denatures proteins to facilitate their precipitation. Methanol is added to complete the denaturing and precipitation process of the proteins. Samples are centrifuged to separate the protein precipitate from the 25OHD and the supernatant loaded onto an Oasis HLB μElution SPE Plate. 25OHD2 and 25OHD3 and their corresponding internal standards are separated from interfering compounds using specific solvent washes and eluted into 96-well plates ready for transfer to the ACQUITY UPLC Sample Manager (SM-FTN) for analysis.
The analytes are separated on a MassTrak Vitamin D BEH Phenyl IVD Column using the ACQUITY UPLC I-Class System prior to the multiple reaction monitoring (MRM) detection and quantitation by the Xevo TQD. The data are acquired using the MassLynx Software and sample concentrations are calculated using TargetLynx Application Manager.
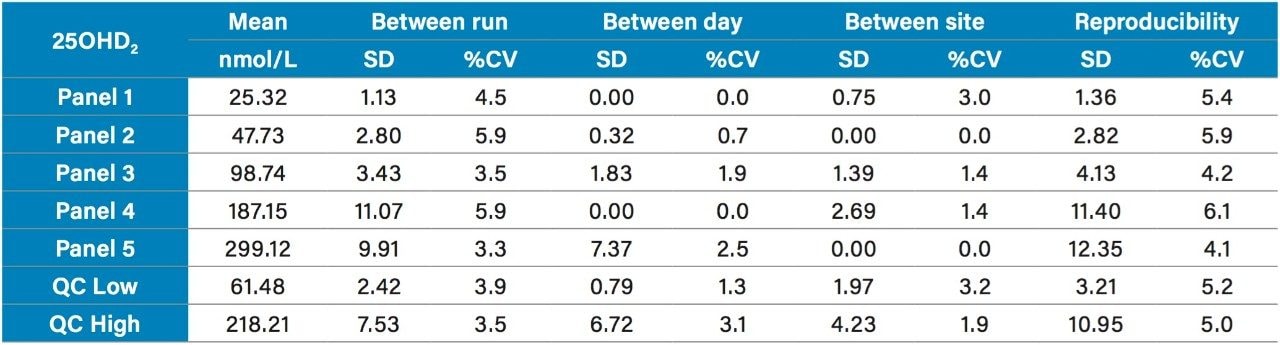
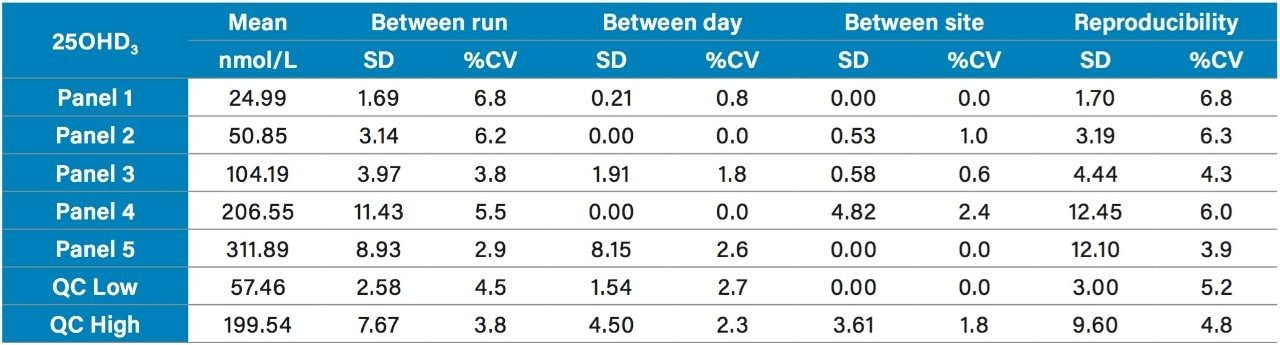
Precision was verified in a multi-site precision evaluation conducted in three sites using a total of three systems, three operators, and a single reagent kit lot according to CLSI EP05-A3. Five serum pools (Panel 1–5) spanning the range of the assay from 22 nmol/L to 300 nmol/L for 25OHD2 and 25OHD3 and the Quality Controls (Low and High) were analyzed in replicates of five, for five days, at three sites, providing a reproducibility of ≤5.9%CV for 25OHD2 (range 4.1–6.1%) and ≤6.8%CV for 25OHD3 (range 3.9–6.8%) as shown in Tables 3 and 4 respectively.
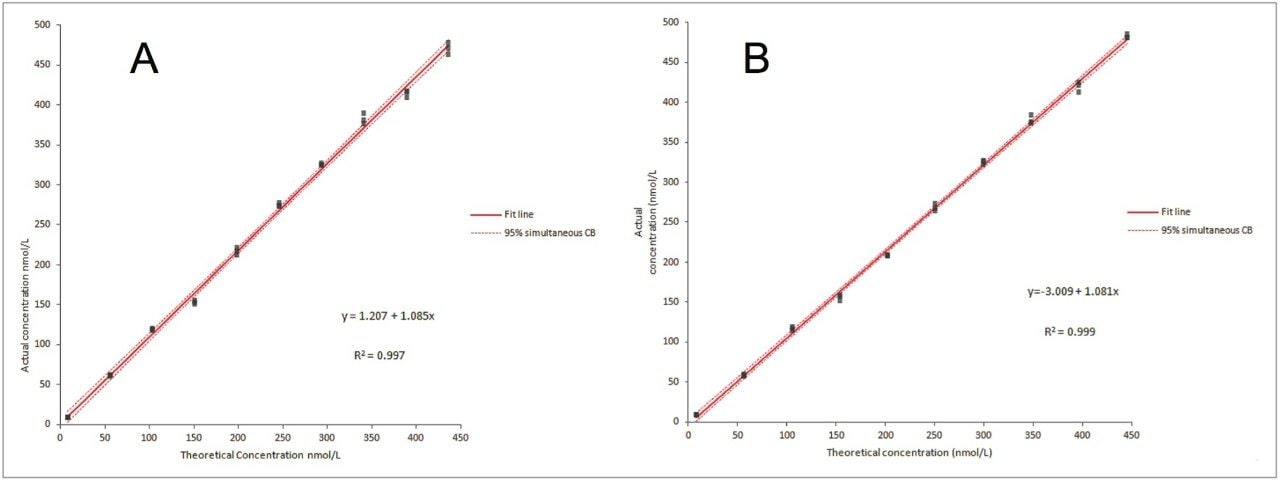
The reportable range for linearity for 25OHD2 and 25OHD3 was demonstrated to be from 10 nmol/L to 375 nmol/L, within a range of ±10% for this interval (Figure 2). Linearity was assessed in a single site study according to CLSI EP6-A.
Sensitivity testing was conducted in accordance with CLSI EP 17-A2 and the Limit of Detection and Limit of Quantification were 2.7 nmol/L and 7.3 nmol/L, respectively, for 25OHD3. The Limit of Detection and Limit of Quantification were 2.7 nmol/L and 5.7 nmol/L, respectively, for 25OHD2.
The carryover of the assay was determined to be 0.87 nmol/L for 25OHD2 and 0.37 nmol/L for 25OHD3. The total carryover effect for 25OHD was 1.24 nmol/L which is lower than the limit of quantitation of the assay.
Interference testing was conducted, according to the procedures described in CLSI EP7-A. Both endogenous and exogenous compounds were tested. Sample collection tube preservatives were tested (potassium-EDTA, sodium citrate, sodium heparin, and lithium heparin) as well as certain vitamin D metabolites (1-alpha-(OH)vitamin D3, 1-alpha-(OH)vitamin D2, 1,25di(OH)vitamin D2, 1,25di(OH)vitamin D3, 3-epi-25(OH)vitamin D2, 3-epi-25(OH)vitamin D3, 23R,25di(OH)vitamin D3, 24R,25di(OH)vitamin D3, 24S,25di(OH)vitamin D3). The mean recovery for 25OHD2 and 25OHD3 was between 85%–115% in the presence of the endogenous, exogenous and vitamin D metabolites tested, with the exception of 3-epi-25OHD2 and 3-epi-25OHD3. Interference was observed from 3-epi-25OHD2 for 25OHD2 and 3-epi-25OHD3 for 25OHD3, which co-elute with the analytes of interest.
A sample dilution protocol using 1:1 proportion of sample to calibrator 0 was developed and demonstrated to provide recovery within 85–115% for out of range samples (>375 nmol/L of 25OHD2 or 25OHD3).
The accuracy of the MassTrak Vitamin D Kit has been demonstrated. Waters enrolls in the VDSCP for total serum 25OHD. The program assesses bias and precision of assays relative to reference measurement procedures. The MassTrak Vitamin D assay achieved a mean bias of 0.6% from the VDSCP reference values and a mean imprecision of 4.9%, meeting the certification performance criteria. The linear regression analysis agreement between mean MassTrak Vitamin D Kit measured concentrations (n=40) and the values assigned by the reference measurement procedure was described by the equation: MassTrak Vitamin D Kit = 1.1143(VDSCP) - 7.5584, with R2= 0.9715.
Finally, metrological traceability of the MassTrak Vitamin D Kit calibrators and quality controls to NIST SRM2972 has been established aiding laboratories in their compliance to ISO 15189 (720005886EN).
The MassTrak Vitamin D Solution provides an LC-MS/MS based clinical diagnostic solution that is CE Marked to the IVDR (EU) 2017/746 for quantitative assessment of vitamin D status in human plasma or serum.
The MassTrak Vitamin D Solution demonstrates excellent precision and linearity across a wide range. The limit of quantitation has been determined to be 7.3 nmol/L for 25OHD3 and 5.7 nmol/L for 25OHD2. The C3-epimers; 3-epi-25OHD2 and 3-epi-25OHD3 were demonstrated to be the only interference in the method. Therefore, in samples containing high concentrations of these C3-epimers, 25OHD2 and 25OHD3 can be overestimated.
In addition, the time consuming sample preparation steps have been automated to minimize operator and transcription errors in sample processing with sample tracking from the sample bar code through to reporting of the result in TargetLynx.
Metrological traceability of commercial calibrators is important to aid compliance to ISO 15189 in your laboratory. The MassTrak Vitamin D Kit calibrators and quality controls are traceable to NIST SRM2972 with uncertainty measurements provided in the Directions for Use (p/n: 715004830IVD) to aid compliance.
Intended Use: The Waters MassTrak Vitamin D Kit is for the quantitative determination of 25-hydroxyvitamin D3, 25-hydroxyvitamin D2, which in combination provide the total 25-hydroxyvitamin D in human plasma and serum using an automated liquid handling system and Waters ACQUITY UPLC I-Class/Xevo TQD IVD System. Results are to be used as an aid in the assessment of vitamin D sufficiency.
For information on availability, please contact your local sales representative.
*Not available from Waters, must be ordered from Tecan.
720005942, Revised April 2022