
This study describes how effectively and efficiently nitroaromatic compounds can be separated on CORTECS Phenyl Columns.
The CORTECS Phenyl Column showed unique selectivity of separation using either acetonitrile or methanol due to its pi-pi bond interactions. The CORTECS C8 Column and CORTECS Phenyl Column are essential tools for developing methods, which can be efficiently and effectively take advantage of different solvents to achieve the best separation.
Nitroaromatic compounds are rarely formed spontaneously in nature. They are mainly synthesized and can be found in dyes, polymers, pesticides, and explosives. The extensive productions of such compounds eventually create environmental issues by contaminating soils and ground waters. The nitro group provides chemical and functional diversity in these molecules and also contributes to the recalcitrance of these compounds to biodegradation. Due to the resistance to oxidative degradation, recalcitrance is further compounded by their acute toxicity, mutagenicity, and easy reduction into carcinogenic aromatic amines.1 They have been listed as hazardous compounds by the Environment Protection Agency (EPA) and are considered hazardous to human health.
This application note explores how effectively and efficiently these compounds can be separated on CORTECS Phenyl Columns. The effect acetonitrile and methanol have on separating these compounds will be investigated. Understanding the effect of solvent selection on selectivity is important not only financially, but also to develop better chromatographic methods. Using a method for calculating solvent strength was used to demonstrate the selectivity difference of acetonitrile and methanol on CORTECS C8 Columns and CORTECS Phenyl Column, 2.7 μm chemistries.2
|
Mobile phase: |
70:30 acetonitrile:water |
|
Separation mode: |
Isocratic |
|
Detection: |
UV 254 nm |
|
Column: |
CORTECS Phenyl Column, 2.7 μm, 2.1 x 100 mm ; CORTECS C8 Column, 2.7 μm, 2.1 x 100 mm |
|
Column temp.: |
40 °C |
|
Weak needle wash: |
10:90 ACN:water |
|
Strong needle wash: |
50:50 water:ACN |
|
Seal wash: |
20:80 ACN:water |
|
Flow rate: |
0.5 mL/min |
|
Injection volume: |
1.0 μL |
|
Mobile phase: |
45:55 methanol:water |
|
Separation mode: |
Isocratic |
|
Detection: |
UV 254 nm |
|
Column: |
CORTECS Phenyl Column, 2.7 μm, 2.1 x 100 mm; CORTECS C8 Column, 90Å,2.7 μm, 2.1 x 100 mm |
|
Column temp.: |
40 °C |
|
Weak needle wash: |
10:90 ACN:water |
|
Strong needle wash: |
50:50 water:ACN |
|
Seal wash: |
20:80 ACN:water |
|
Flow rate: |
0.5 mL/min |
|
Injection volume: |
1.0 μL |
|
Vials: |
TruView LCMS Certified Clear Glass 12 x 32 mm Screw Neck Max Recovery Vial, with Cap and Preslit PTFE/Silicone Septa, 1.5 mL Volume |
|
QC Reference Materials: |
Neutrals QC Reference Material |
|
Data management: |
Empower 3 CDS |
The nitroaromatic compounds were prepared as 10 μg/mL solutions in 100% methanol. The sample was transferred to a TruView LCMS Certified Clear Glass 12 x 32 mm Screw Neck Max Recovery Vial, with Cap and Preslit PTFE/Silicone Septa, 1.5 mL Volume for injection.
In this application, the selectivity based on solvent selection when the separation is performed on CORTECS Phenyl Column, 2.7 μm, 2.1 x 100 mm and the CORTECS C8 Column, 2.7 μm, 2.1 x 100 mm will be investigated to effectively and efficiently analyze nitroaromatic compounds. Waters Neutrals QC Reference Material was used as a benchmark for selectivity for the CORTECS C8 and Phenyl 2.7 μm Columns. When acetonitrile was used as the strong solvent, baseline resolution was achieved for the Neutral QC Reference Material compounds on both columns in total separation times of less than one minute (See Fig. 1). Figure 1 show how very similar the CORTECS C8 and Phenyl Columns are in terms of hydrophobicity. The CORTECS C8 shows slightly higher solubility of the hydrophobic molecules (naphthalene and acenaphthene) in the stationary phase due to the alkyl chains.
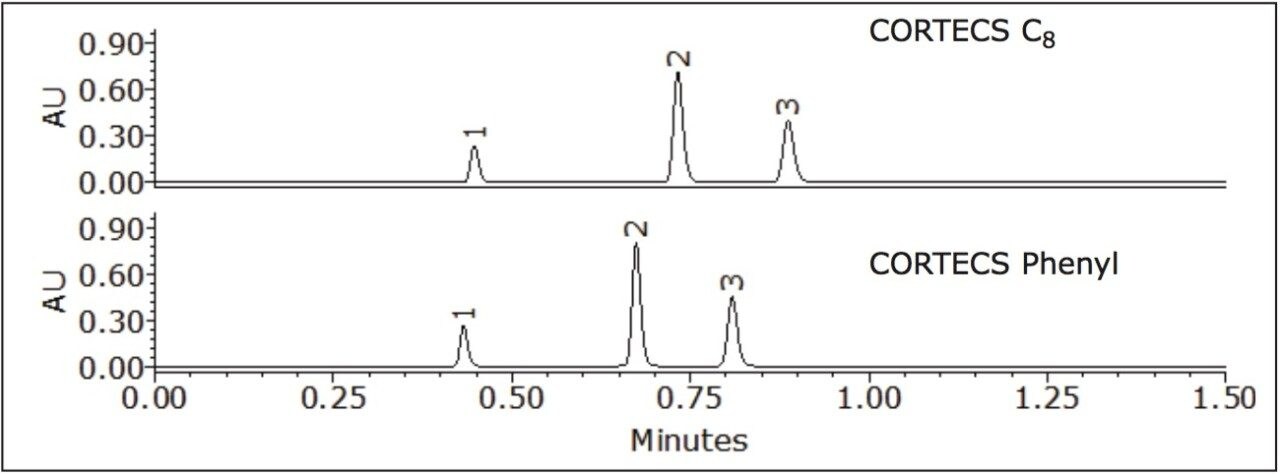
The effect acetonitrile has on the separation of nitroaromatic compounds using CORTECS C8 and Phenyl 2.7 μm Columns was investigated (see Figure 2).
The selectivity of the CORTECS Phenyl Column for peaks 1 and 3 is different from the CORTECS C8 Column. Peaks 1 and 2 were also more retained on the CORTECS Phenyl Column than the CORTECS C8 Column. Separation on the CORTECS Phenyl Column was superior to the CORTECS C8 Column due to pi-pi (π-π) interactions of the ligand and analyte. The selectivity on the CORTECS Phenyl Column between peak 3 and 2 was 1.16 and peak 2 and 1 was 1.34. In theory, an acceptable value for selectivity should be >1.0.3
To enhance pi-pi effects, aprotic acetonitrile was replaced by protic methanol and selectivity of the nitroaromatic compounds on CORTECS C8 and Phenyl 2.7 μm Column was evaluated. Equation 11 below was used to determine the appropriate concentration of methanol to match the solvent strength of acetonitrile. Ensuring peaks are baseline separated is important to successful use of this equation, which is the case (see Figure 2) is for CORTECS Phenyl Column using 40% acetonitrile.
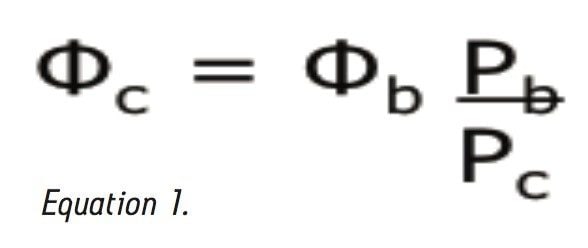
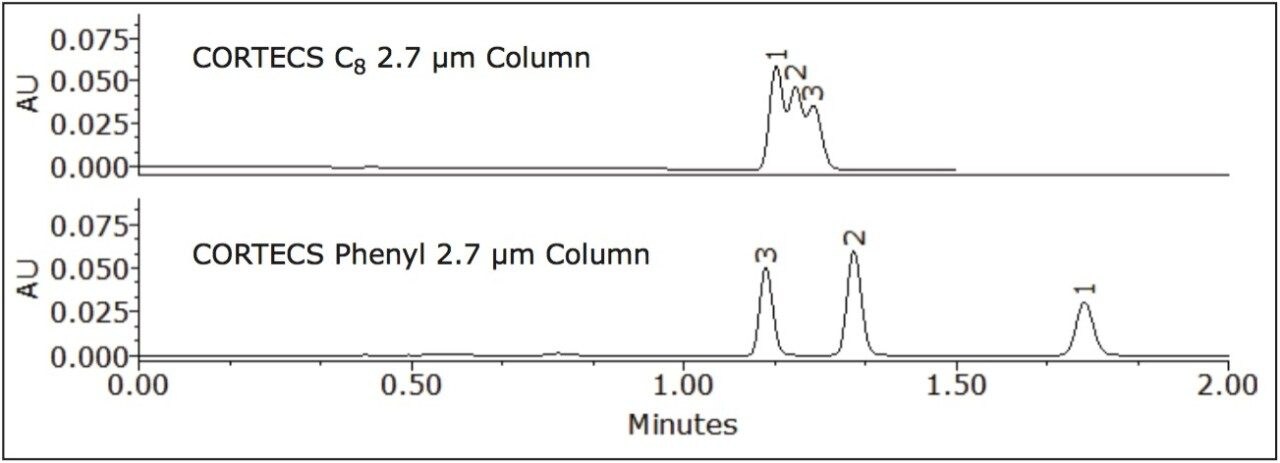
Where Φc is the percent methanol needed, Φb is the percent acetonitrile; Pb is the P' of acetonitrile (5.8) and Pc is the P' for methanol (5.1). The P' values were obtained from the properties of solvents for use in liquid chromatography2 and correspond to the specific polarity of each solvent. Φc was calculated to be 45% methanol. The data table and chromatogram below shows the separation using methanol as the strong solvent (see Table 1 and Figure 3).
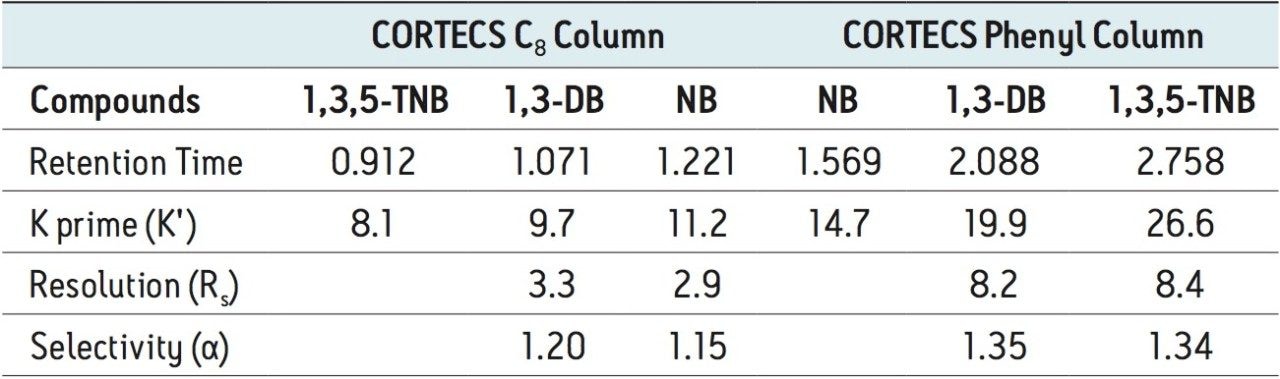
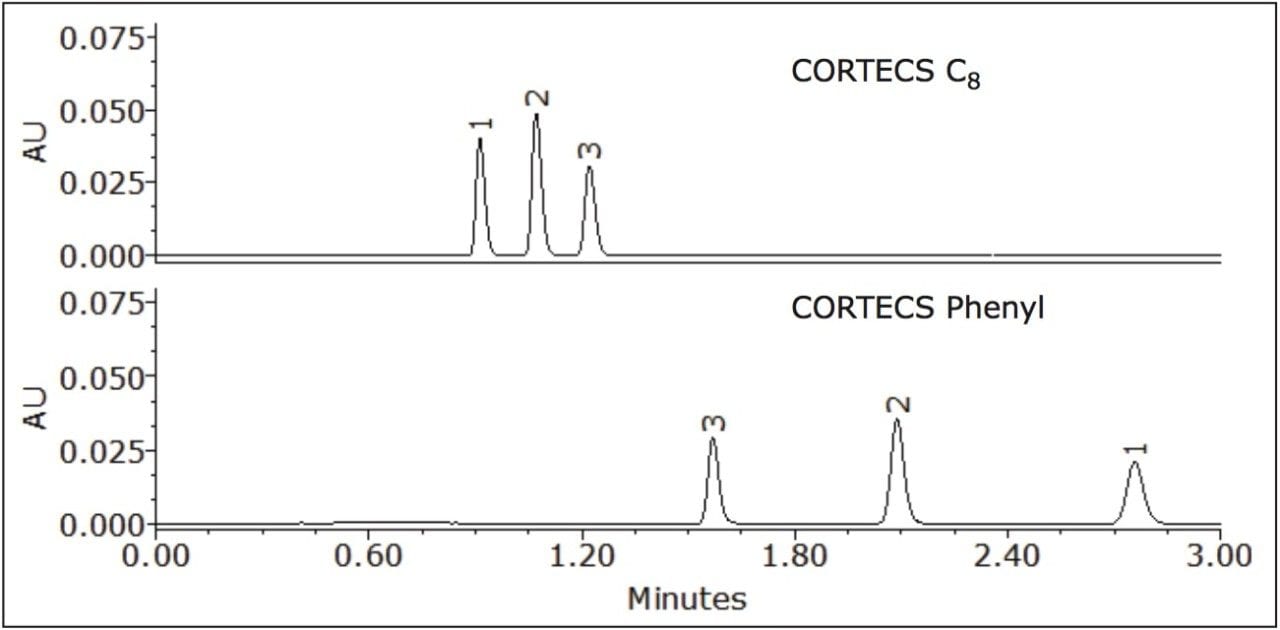
The three compounds were separated successfully using methanol as mobile phase and the peaks were baseline resolved. The retention of the compounds remained nearly the same on the CORTECS C8 Column, with a slight change in selectivity. However, a significant enhancement of resolution was observed on the CORTECS Phenyl Column using methanol rather than acetonitrile. The protic property of methanol makes it much more favorable for aromatic compounds separations than the aprotic properties of acetonitrile (as seen in Figure 2). The data (Table 1) shows that the Rs>1.5 and α>1.0 for both columns. Using methanol as mobile phase enhances selectivity than using acetonitrile
Two standard mixtures of nitroaromatics, EPA 8330 Mix A and Mix B, related to the manufacturing of explosives and their degradation products, were tested on the CORTECS C8 Column and the CORTECS Phenyl Column. The analytes are shown in Figure 4, where Mix A comprises of peaks 1 through 8 and Mix B comprises of peaks 9 through 14. These standards were transferred to a TruView LCMS Certified Maximum Recovery Vial and analyzed using mobile phase 45:55 methanol:water on a CORTECS C8 Column and a CORTECS Phenyl Column (see Figure 4 below).
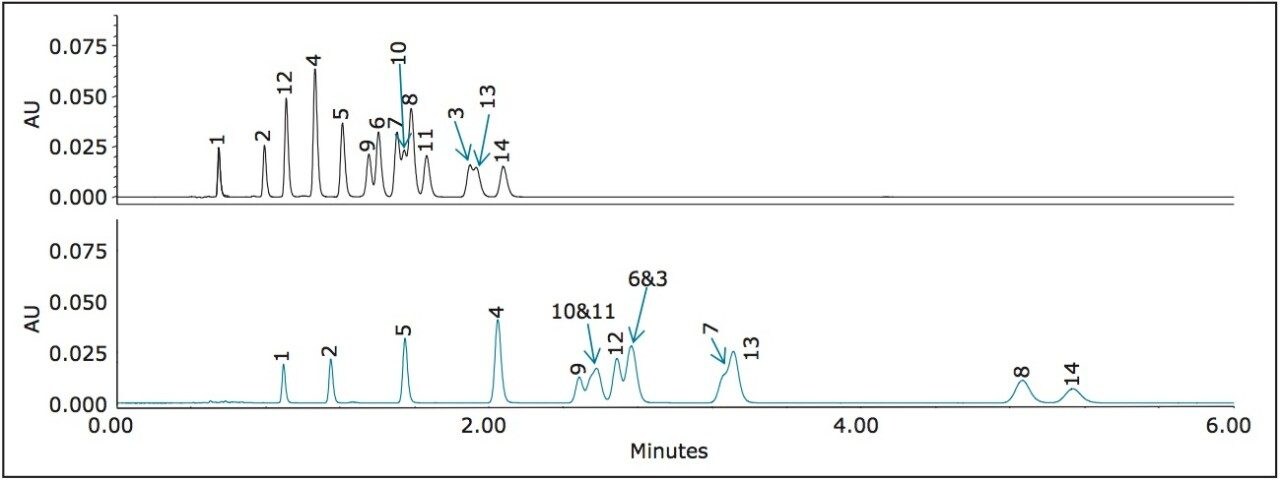
The use of CORTECS Phenyl Columns and a protic mobile phase results in a better separation of nitroaromatic compounds than on the CORTECS C8 Column. The enhanced selectivity afforded by phenyl ligands not only increases the separation window, but dramatically improves the resolution between many of the peaks. For instance, peak 8 becomes completely resolved from peaks 7, 10, and 11 in Figure 4. This separation could be optimized further by the use of a shallow gradient of methanol.
Although the choice of stationary phase plays a role in influencing the selectivity of separation, mobile phase plays an equal role. Acetonitrile is often preferred for reverse phase liquid chromatography, but methanol can be a high selectivity alternative. Using acetonitrile did not separate the nitroaromatic compounds using the CORTECS C8 Columns, while methanol provided a unique selectivity for these compounds on CORTECS C8 Columns and CORTECS Phenyl Columns due to its protic property. The CORTECS Phenyl Column showed unique selectivity of separation using either acetonitrile or methanol due to its pi-pi bond interactions. Performing solvent selection studies for isocratic separations, Snyder’s equation2 is an important tool to use in order to optimize selectivity and enhance analysis time. The CORTECS C8 Column and CORTECS Phenyl Column are essential tools for developing methods, which can be efficiently and effectively take advantage of different solvents to achieve the best separation.
720005580, January 2016