This is an Application Brief and does not contain a detailed Experimental section.
Recently there has been a trend towards LC-MS for the bioanalytical quantification of peptide and protein therapeutics due to the many benefits it affords (multiplexing, improved specificity, broader dynamic range and fast method development time). However, protein quantification challenges still exist with regard to assay sensitivity, small sample volume requirements, and laborious and often complex workflows. This is especially in contrast to ligand binding assays. Thus, there is a strong need for simpler and more broadly applicable standardized workflows, ideal for low sample volume assays (<50 μL), for the quantification of proteins.
Broad applicability across a range of sample volumes, and the inherent flexibility of the ProteinWorks eXpress Direct Digest Kits for the quantification of monoclonal antibody (mAb) drugs by LC-MS/MS.
Over the past decade, MS platform technologies have steadily increased in sensitivity and ease of use. At the same time, this increase in sensitivity has facilitated the use of smaller sample volumes. As such, microsampling techniques have become common place. These techniques allow for collection from pre-clinical species such as rodent, and minimize animal use. Additionally, as biological drug development increases, LC-MS assays to quantify both therapeutic and endogenous peptides and proteins are growing. The desire to use small sample volumes presents an even greater challenge for protein and peptide quantification analyses due to the complexity of the workflow and their inherent lower MS sensitivity, relative to small molecules. While some studies are dominated by the need to use sample volumes <50 μL, others require more sample to increase sensitivity. Therefore, there is a need for a simple, kit-based approach that accommodates a range of plasma volumes and is easily implemented by scientists unfamiliar with the workflows.
LC-MS for quantification of therapeutic and endogenous proteins is steadily gaining in its use, and unlike small molecule LC-MS analysis, it presents many new and unique challenges. Typically one needs to perform an enzymatic digestion (most commonly trypsin) in plasma, followed by quantification of one or multiple representative tryptic peptides using multiple reaction monitoring (MRM). There is no single standard work flow for this task, and it is often traditional “small molecule” scientists faced with learning and implementing these unfamiliar workflows, which can be complex and laborious. Required sample volumes will vary depending on assay or sensitivity requirements. This can make it difficult to know where to start, particularly if one considers the diversity of proteins, as they can vary greatly in their size, structure, and amino acid sequence. For this reason, we have developed a fully flexible yet generic, kit-based sample preparation strategy using the ProteinWorks eXpress Direct Digest Kit (p/n 176003688) for the simultaneous quantification of the monoclonal antibody drugs: infliximab, adalimumab, bevacizumab, and trastuzumab with plasma volumes ≤70 μL. In short, the mAb’s were spiked into plasma at concentrations between 5–50 μg/mL. Sample aliquots of 15, 35, and 70 μL of the mAb spiked plasma were then directly digested with the ProteinWorks eXpress Direct Digest Kit using the generic protocol provided. For all plasma sample volumes, and using the ProteinWorks kits for digestion, standard curves for all peptides, from all mAb drugs, were linear with R2 ≥0.99, using 1/x weighting. Mean % accuracies of the standard curve points were >99% (Table 1). Standard curves arising from a range of starting plasma volumes are shown in Figure 1, Panels A–C. The representative infliximab tryptic peptide (DILLTQSPAILSVSPGER) is shown as an example. The chromatographic performance is highlighted in Figure 2, panels A–D. Multiple calibration levels from representative signature peptides of infliximab, bevacizumab, trastuzumab, and adalimumab are shown.
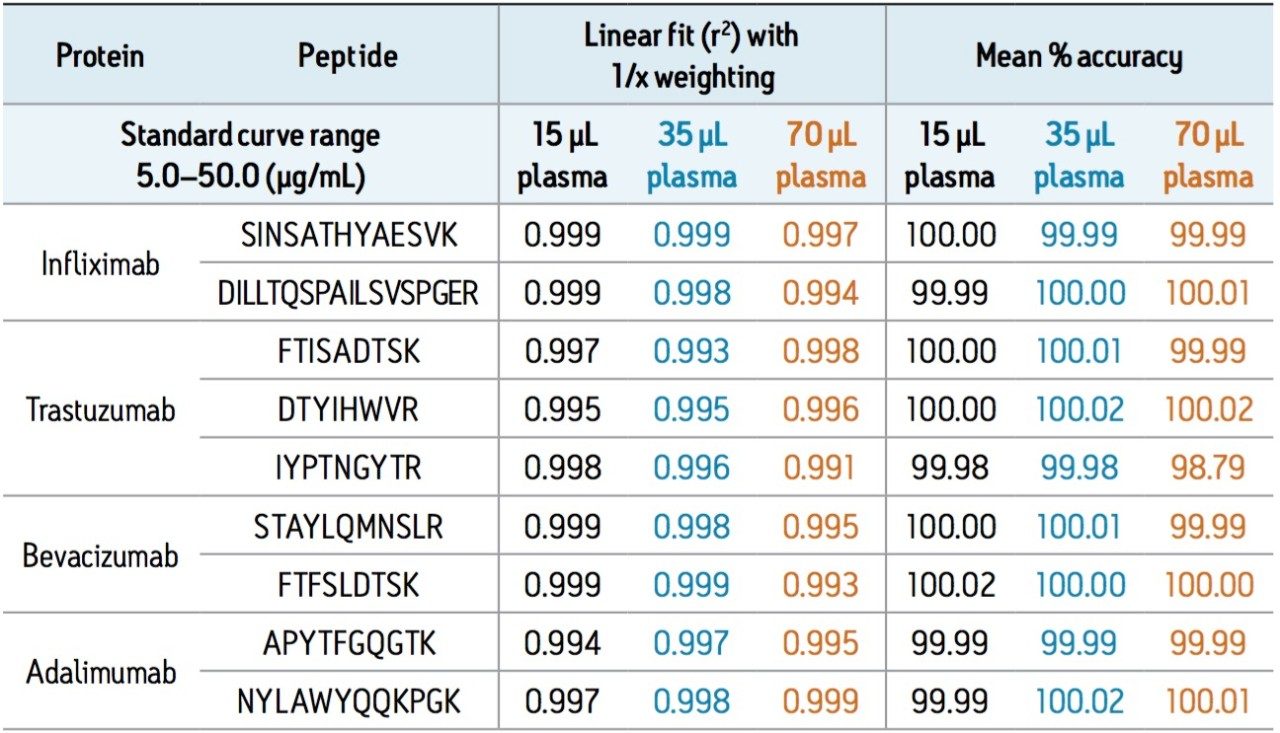
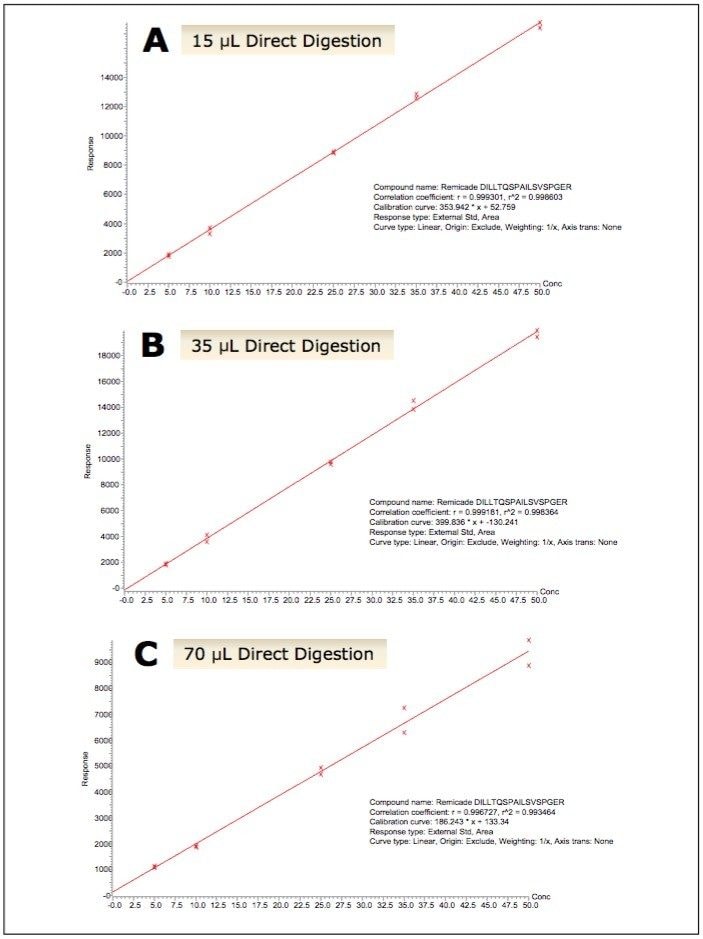
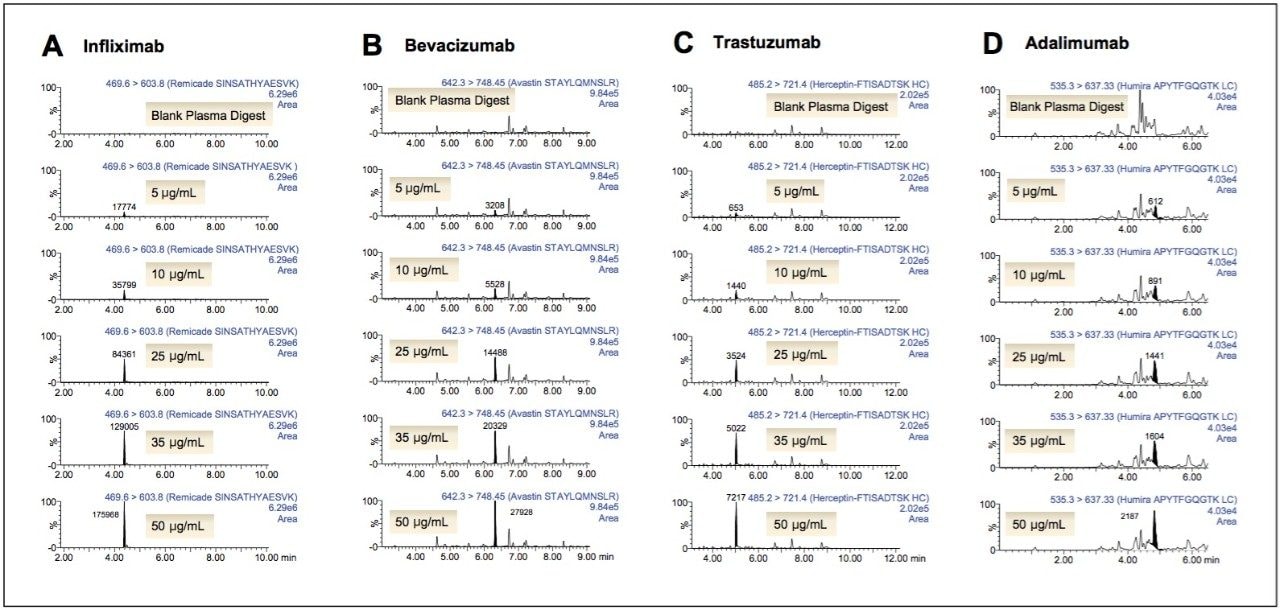
In this study, a flexible ‘kit-based’ approach, using a single protocol which accommodates a range of sample volumes (15–70 μL) eliminated the need for method development in discovery studies, and facilitated the accurate quantification of 4 monoclonal antibody drugs.
720005575, January 2016