
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates a simple workflow for the calculation of clearance PK values using WinNonlin from microsomal incubations acquired and processed using UNIFI Scientific Information System.
UNIFI supports completely customizable data transfer with a variety of third-party data packages. Enhanced export of tables supports communication with virtually any other calculation and statistical software package that support Microsoft Excel formats.
Generating LC-MS data is an integral part of drug metabolism pharmacokinetic (DMPK) departments. How we convert this data to drive decisions is often the bottleneck as many different software tools are used in a typical DPMK lab. It is critical for LC-MS software to support easy transfer of results to a variety of third-party software packages in order to extract and provide key information to support drug discovery and development.
An example of this is to use LC-MS data to calculate pharmacokinetics parameters using industry-standard software packages such as Phoenix WinNonlin (Certara, St. Louis, MO, U.S.). Calculation of PK parameters are commonly performed to establish the viability of a drug candidate or series and help prioritization efforts. Electronic and customizable data transfer/integration between instrumentation software and third-party software platforms (WinNonlin, Spotfire, etc.) is the preferred approach and is essential for handling and processing large amounts of data with both accuracy and speed.
Enhanced export using UNIFI Software provides the following features:
The exported data can be brought into third-party packages without manual copying or transcription and the table layouts preferences can be saved within UNIFI. All of these features help to minimize errors and enable the more confident analysis of large data sets. Here, we provide a step-wise illustration of how to perform a calculation of microsomal clearance from UNIFI data acquired on Xevo G2-S QTof using WinNonlin.
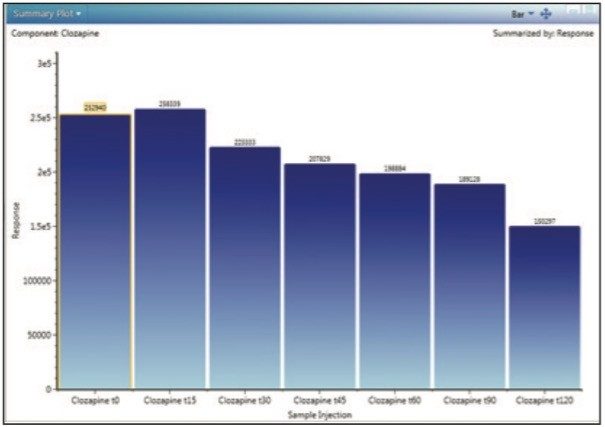
Clozapine incubations were performed using human liver microsomes at 1 mg/mL protein concentration. Incubations were monitored for 2 hours to obtain a time-course study (aliquots withdrawn at 15-minute intervals). Samples were acquired and processed using an ACQUITY UPLC I-Class System and a Xevo G2-S QTof mass spectrometer under UNIFI instrument control. Figure 2 shows a typical profile with parent drug, Clozapine, disappearing over the 2-hour time course as displayed in UNIFI. The data were exported out of UNIFI and imported into WinNonlin (version 6.3) for the calculation of clearance. The following steps illustrate the process.
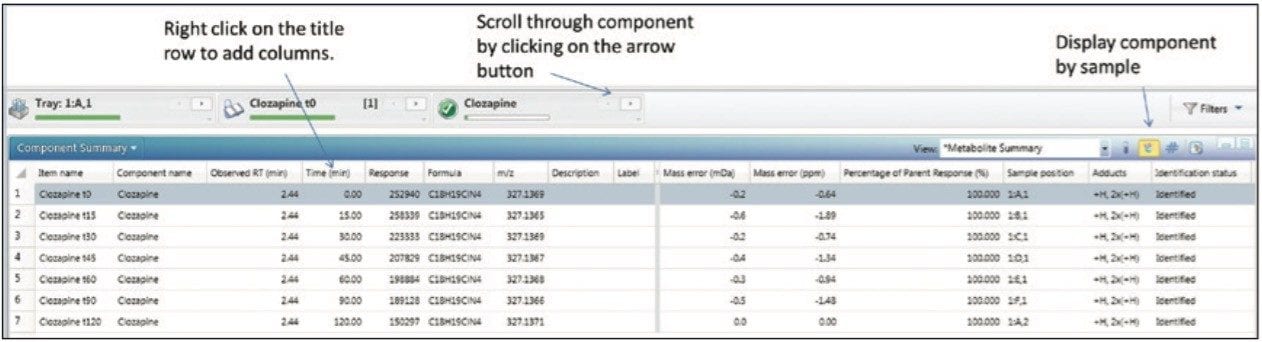
High Resolution Mass Spectrometry (HRMS) data contains both full qualitative and quantitative information. For clearance calculations, only the parent compound information is desired. A table is arranged, filtered, and pivoted which shows the information exactly as we wish to export it (shown in Figure 3). If additional columns are needed for export or columns need to be removed, this is modified by right clicking on the title row and choosing Add (or Remove) Column. The add/remove column panel will appear allowing the user to modify the field displayed in the table (not shown).
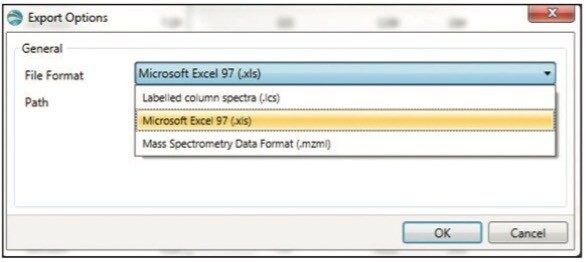
The data shown in the component summary table is then exported by choosing File > Export Filtered Result at the top right corner (not shown). A dialog box called Export Option appears (Figure 4). Choose Microsoft Excel as the file format and enter a filename and folder destination to complete the export. If desired, the data can also be exported using copy (by right-clicking within a table in UNIFI and choosing copy in the drop-down menu) and directly paste into other software packages with Excel-like functionality.
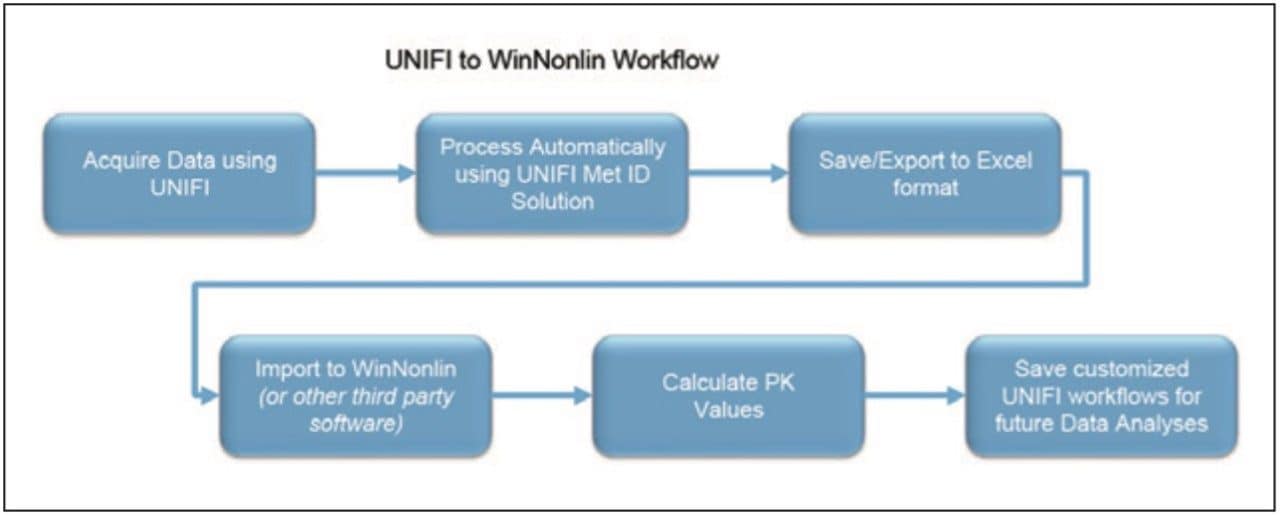
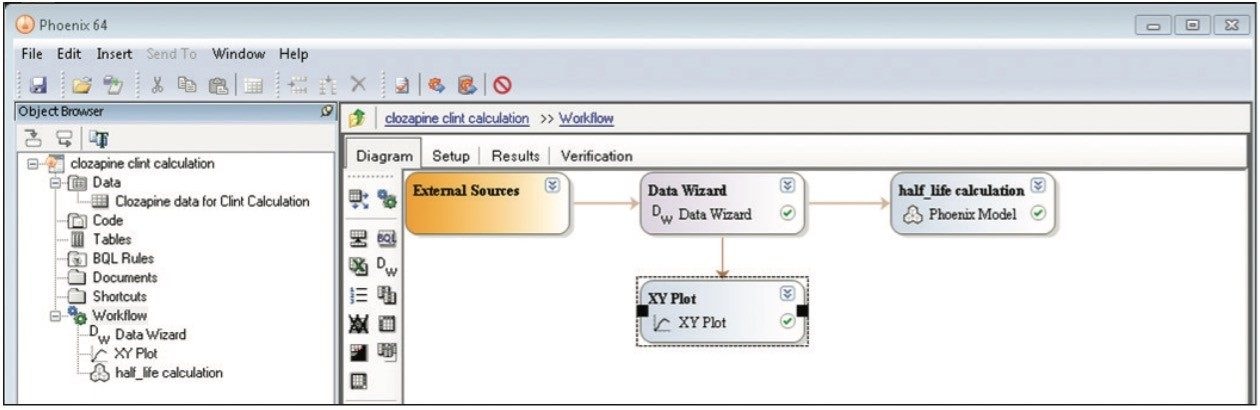
A simple workflow for clearance estimation is shown.
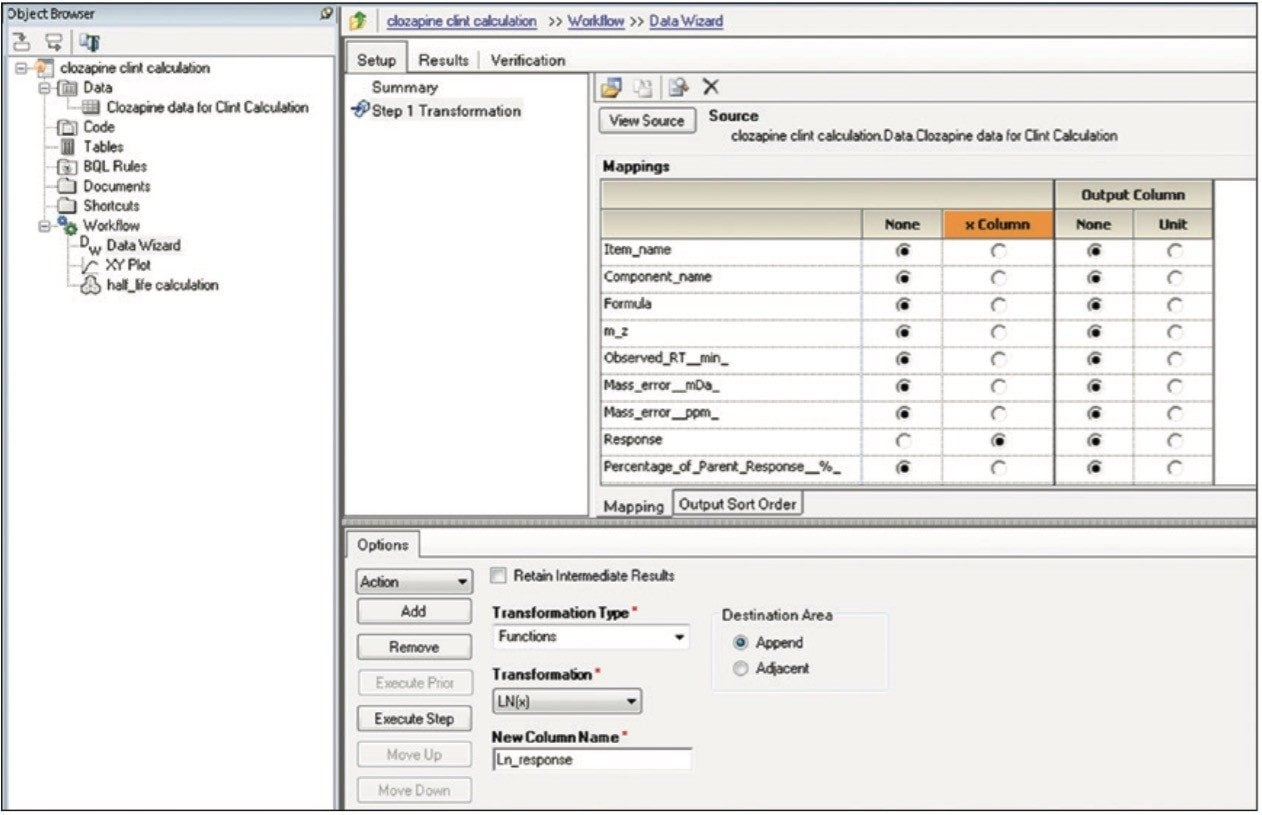
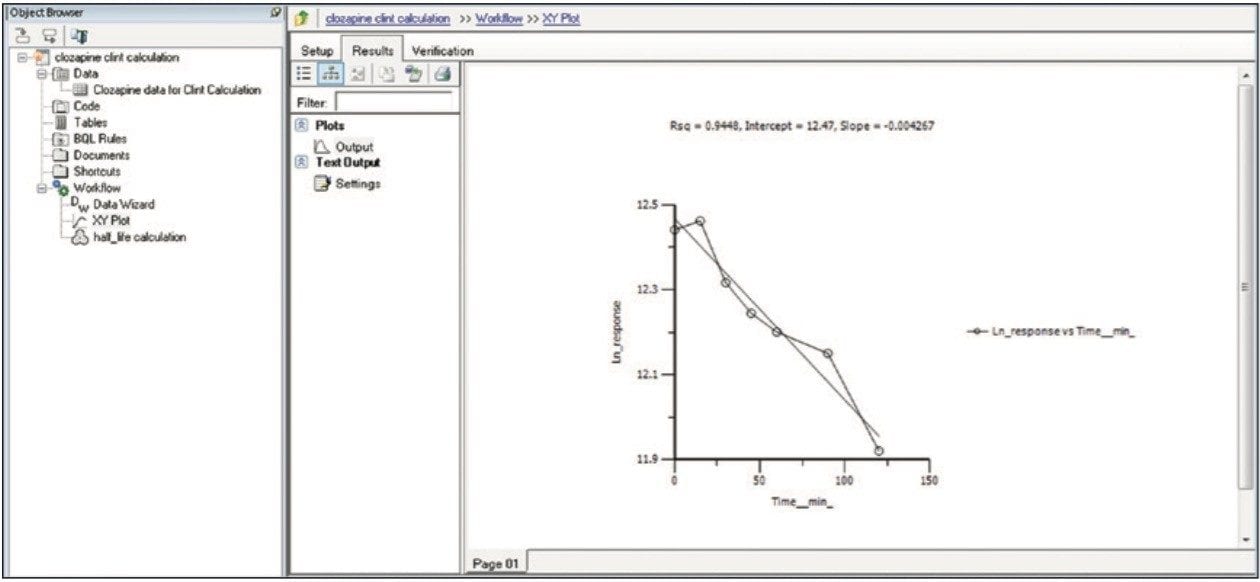
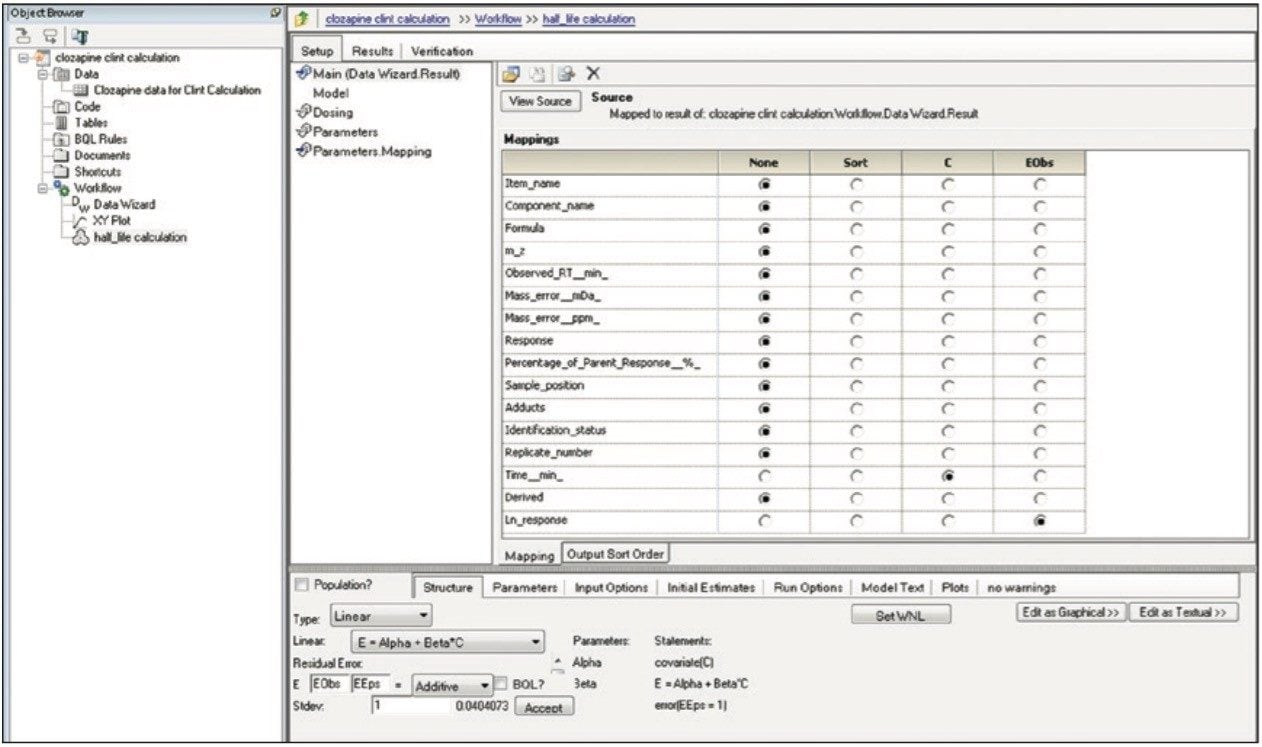
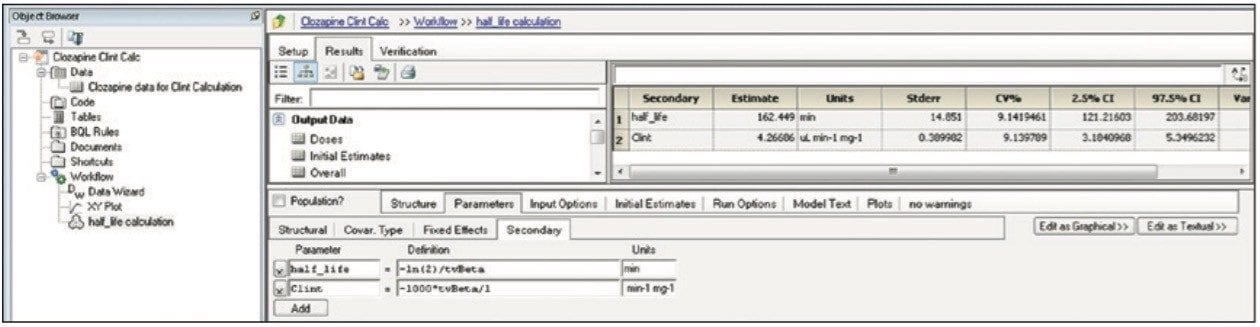
Finally, the above steps are captured by WinNonlin as a workflow shown in Figure 9. The workflow can be saved as a template and applied to future datasets.
Transferring data between software packages can be a bottleneck and is prone to errors if done manually. This technology brief illustrates that data acquired and automatically processed using UNIFI Software can be easily exported for calculations using third-party software solutions, providing both a simple and powerful solution for a DMPK department. By combining workflows in UNIFI and WinNonlin, the user can now easily import and process future datasets from UNIFI and then save these data views for future analyses. This is demonstrated with a calculation using WinNonlin for the estimation of clearance value of clozapine in human liver microsomes.
720004947, February 2014