
This application note demonstrates the use of High Definition Mass Spectrometry (HDMS) as an important method development tool to support the unequivocal identification of fluoroquinolone antibiotics in crude tissue extract.
The fluoroquinolones are a class of antimicrobial agents that have been administered to livestock for different purposes that include: (a) prevention and control of infections, and (b) growth promotion. Due to the concerns regarding the spread of resistant microorganisms in the human population, the U.S. Food and Drug Administration (FDA) introduced a ban on the use of enrofloxacin and ciprofloxacin in livestock production in September, 2005.1,2 The use of antibiotic growth promoting agents (AGPs) in animal husbandry has been forbidden in the European Union (EU) since 2006, when the final four antibiotics were banned as growth promoters.3
The fluoroquinolones are chemically diverse zwiterionic species, although all posses a basic 4-quinolone ring structure. Various modifications, involving substitution of different functional groups around the quinolone ring (benzopyridone nucleus) have been made to improve the antimicrobial potency and the pharmacokinetic properties. The fluoroquinolones have a fluorine atom at position six on the bicyclic ring structure (Figure 1), and show an expanded spectrum of microbiological activity. EU Maximum Residue Levels (MRLs) currently exist for eight (fluoro)-quinolone compounds ranging from 10 to 1900 μg kg-1 dependant on the species and tissue type.4
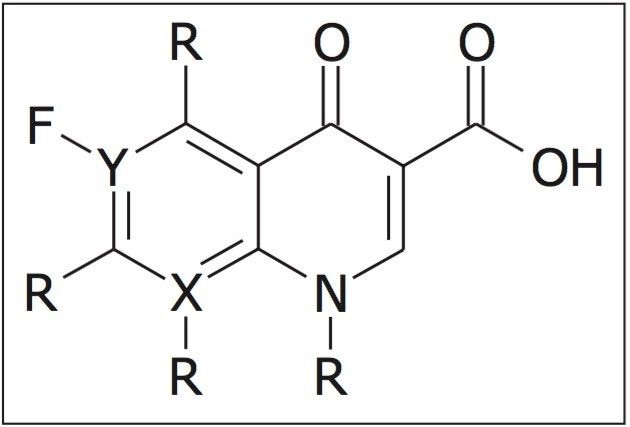
The typical requirements for fluoroquinolone residue analysis are to employ a solvent extraction step, followed by solid phase extraction (SPE) purification and detection using LC coupled to UV, with fluorescence (FL) or mass spectrometry (MS) detection. These methods are often limited to the detection of a small number of target analytes and have low sample throughput.5 Many different types of mass analyzers are routinely utilized for veterinary drug residue (VDR) analysis, including single quadrupole, tandem quadrupole, ion trap, and more recently time-of-flight (Tof) based technologies.6,7,8 Tandem quadrupole mass spectrometry has gained widespread acceptance for quantitative analysis over single-stage mass spectrometry because it can provide significant performance benefits in terms of selectivity and sensitivity. This is attributed to the multiple reaction monitoring (MRM) mode, where a precursor ion is mass selected using the first quadrupole, fragmented in a collision cell when a specific product ion is mass selected and using the second quadrupole for detection. Even with this approach, there is still a small probability that other compounds, unrelated to the analyte, will produce a signal. For this reason, a second MRM transition is monitored and the presence of a compound is only considered to be confirmed if both transitions produce chromatographic peaks with retention times corresponding to that of the investigated analyte in pure standard. In addition, the ratio of the intensities of the two recorded MRM traces have to be equal to those obtained for the analyte in pure standard. This concept has been further refined in the European Commission Decision (2002/657/EEC) which regulates the requirements for analytical methods used to quantify and confirm VDRs in food and animal feed.9
Tandem quadrupole instruments are widely used in residue monitoring programs when low detection limits (typically low μg kg-1 concentrations) are required to be achieved in complex matrices. When choosing to implement a new tandem quadruple-based method for VDR analysis, the selection of the most appropriate MRM transitions is critical and must be performed and validated in accordance with the 2002/657/EC guidelines as previously noted.
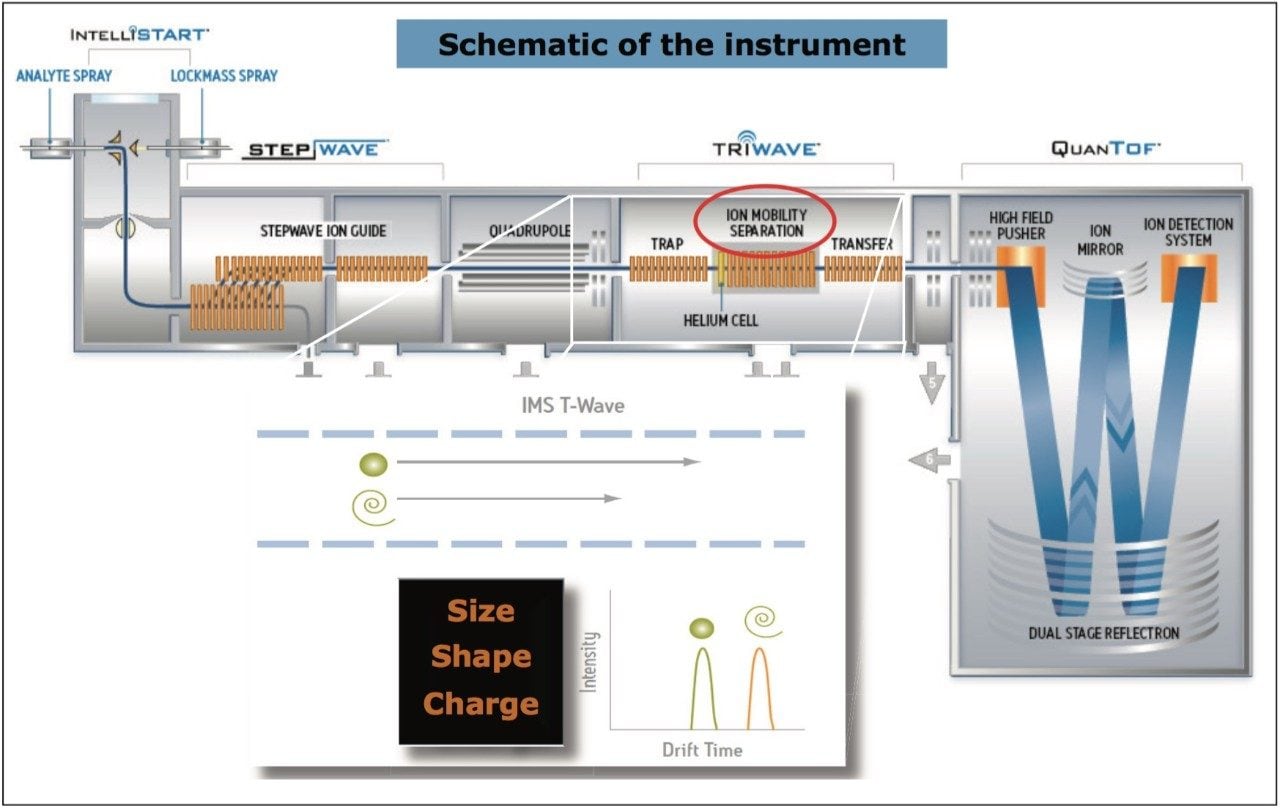
This application note explores the use of High Definition Mass Spectrometry (HDMS) as an important method development tool to support the unequivocal identification of fluoroquinolone antibiotics in crude tissue extract. HDMS has been utilized to analyze crude extracts of porcine muscle tissue to determine the presence of antibiotic residues including the fluoroquinolone class. This technique offers some unique advantages for profiling complex matrices. It uses a combination of high resolution mass spectrometry and high efficiency ion mobility-based measurements and separations. Ion mobility spectrometry (IMS) is a rapid, orthogonal, gas phase separation technique that allows another dimension of separation to be obtained within an LC timeframe. Compounds can be differentiated based on size, shape, and charge. In addition, both precursor ion and fragment ion information can be acquired in a single injection in an HDMS experiment, referred to as HDMSE.
The extracts of porcine muscle tissue were kindly provided by RnAssays for the purpose of this study. Briefly, known blank porcine muscle was fortified with 25 different antimicrobial compounds (from the fluoroquinolone, tetracycline, and amphenicol classes) at the levels relevant to the EU MRL concentrations prior to extraction. The tissue samples were mechanically homogenized in the presence of an aqueous/ organic extraction solvent, followed by a centrifugation step. An aliquot of the supernatant was removed and placed in autosampler vial for subsequent LC-MS analysis.
|
System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 50 x 2.1 mm |
|
Column temp.: |
40 °C |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
Water (0.1% formic acid) |
|
Mobile phase B: |
MeCN (0.1% formic acid) |
|
Injection volume: |
10 μL |
|
Time (min) |
Flow rate |
%A |
%B |
|---|---|---|---|
|
Initial |
0.600 |
95.0 |
5.0 |
|
1.00 |
0.600 |
95.0 |
5.0 |
|
8.00 |
0.600 |
5.0 |
95.0 |
|
9.00 |
0.600 |
95.0 |
5.0 |
|
Mass spectrometer: |
SYNAPT G2-S |
|
Ionization mode: |
ESI positive at 2.0 kV |
|
Cone voltage: |
25 V |
|
Desolvation temp.: |
550 °C |
|
Reference mass: |
Leucine enkephalin, [M+H]+ =556.2771 |
|
Acquisition range: |
50 to 1200 m/z |
|
Acquisition rate: |
4 spectra/s |
|
Collision energy: |
15 to 45 eV |
|
Resolution: |
20,000 FWHM |
|
IMS T-Wave velocity: |
550 m/s |
|
IMS T-Wave pulse height: |
40 V |
|
Drift gas: |
N2 and CO2 |
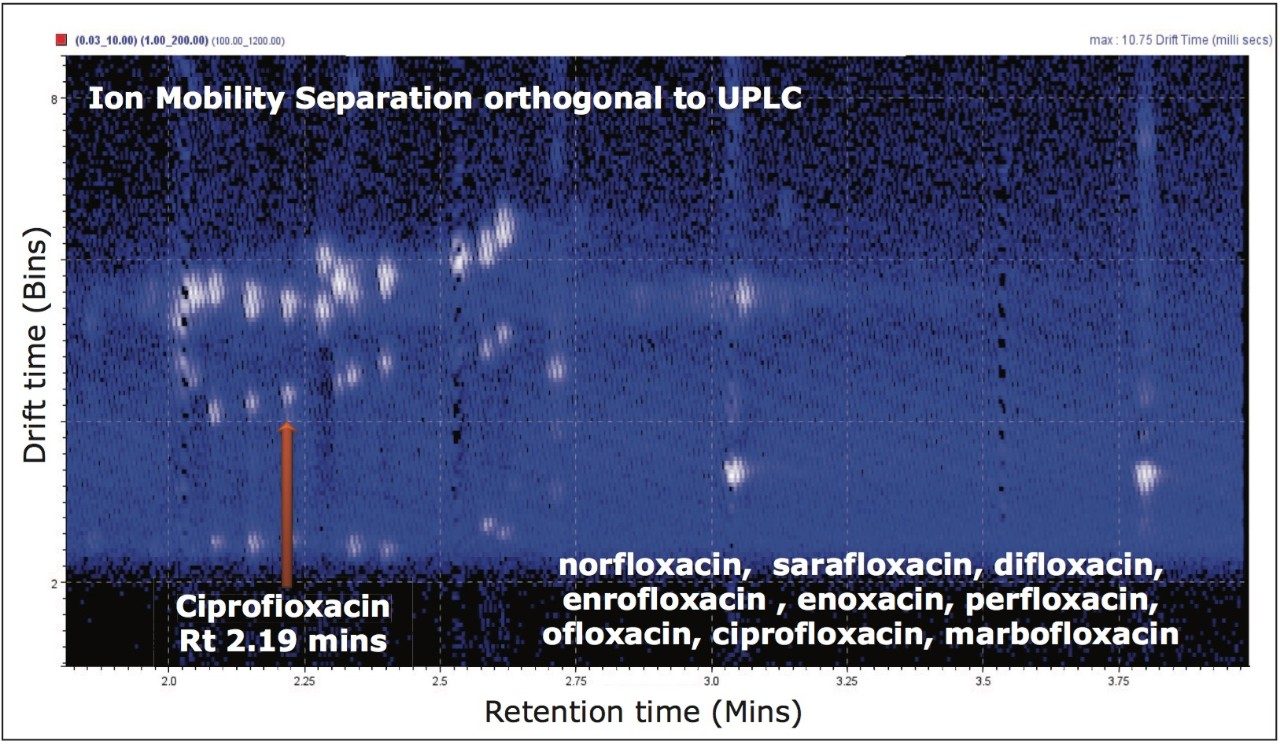
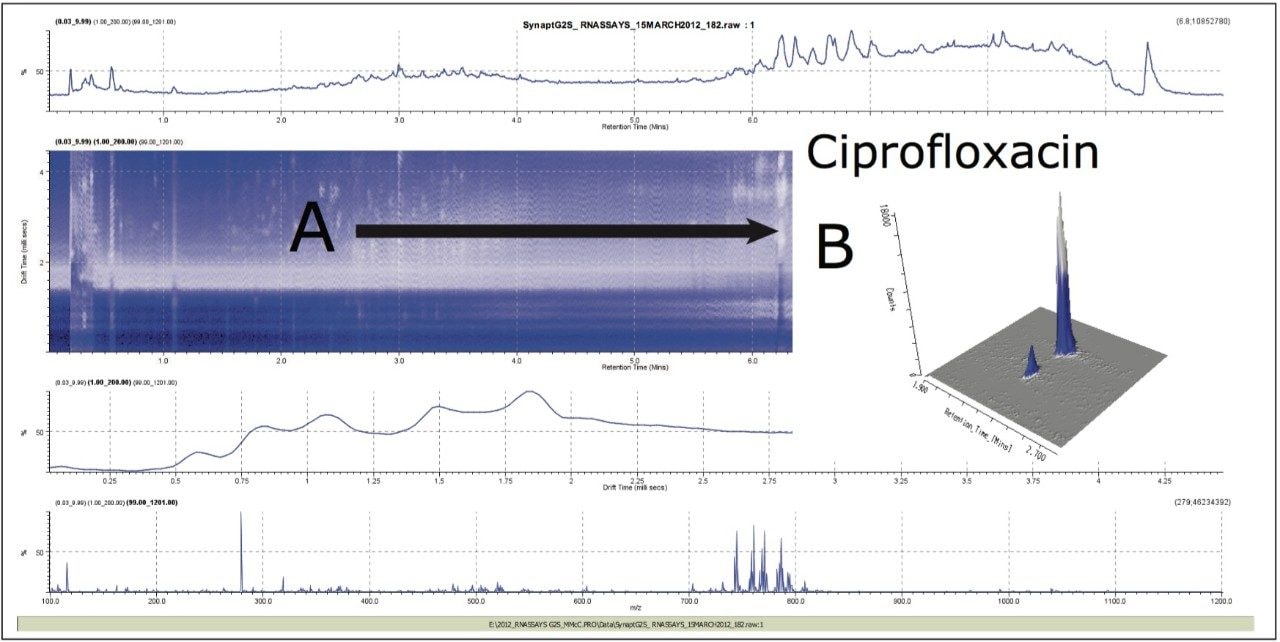
The antibiotic, ciprofloxacin, was determined to elute at a retention time of 2.19 minutes using the generic gradient conditions employed, as shown in Figure 3 where the base peak ion chromatogram is presented.

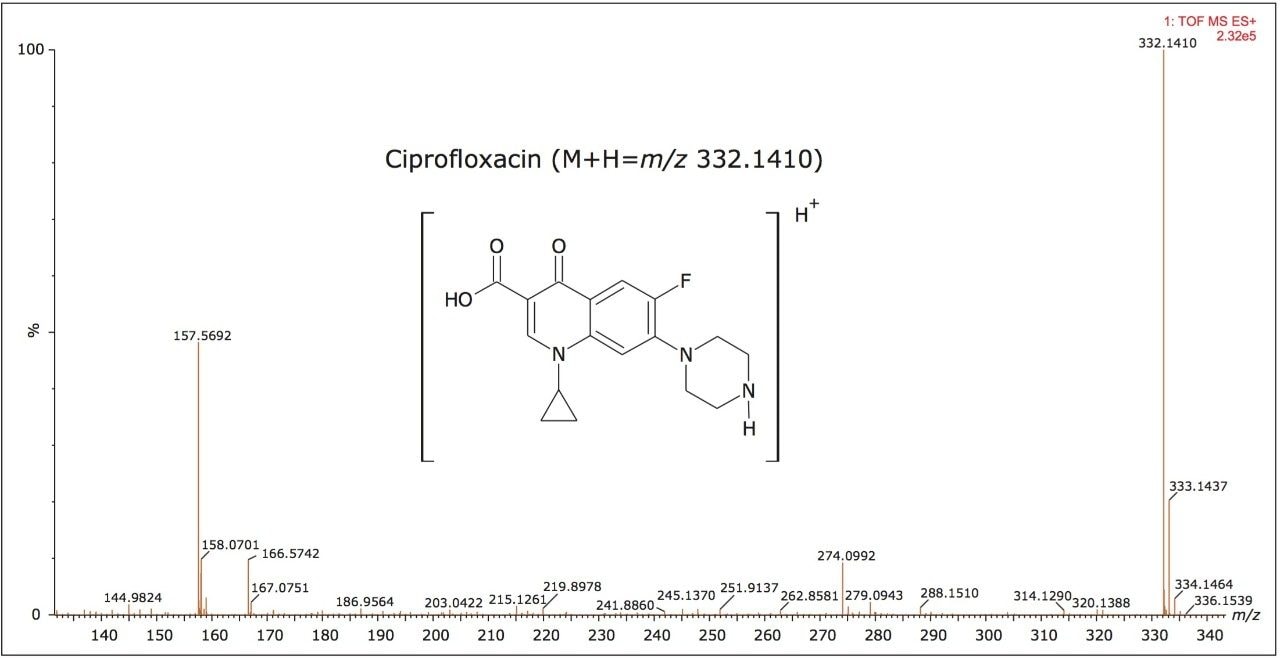
The resultant conventional accurate mass spectrum generated within MassLynx Software is shown in Figure 4, where accurate mass measurement with zero ppm mass error was observed for the [M+H]+ species at m/z 332.1410. The accurate mass measurement obtained and resultant elemental composition generated using the elemental composition calculator within MassLynx enabled complete confidence in the identification of the chromatographic peak at 2.19 minutes.
The conventional data of Figures 3 and 4 suggest the fluoroquinolones analyzed are single components. However, analysis using ion mobility reveals that the fluoroquinolones are comprised of two ionized species, as shown in Figures 5 and 6. These gas phase components, although they only differ with site of protonation, in the case of ciprofloxacin have been separated by 1.14 milliseconds using ion mobility. The mobility separation can be seen within DriftScope in Figure 5, where the plot of drift time versus retention time for a mixture of veterinary drug standards is shown. Figure 5 shows the drift time separated, and multiple ion intensities obtained for the all fluoroquinolones analyzed. In the case of ciprofloxacin, a pair of ion intensities is highlighted. More specific data for this pair are shown in Figure 6, where the respective sites of acid/basic group protonation and drift times are highlighted.10
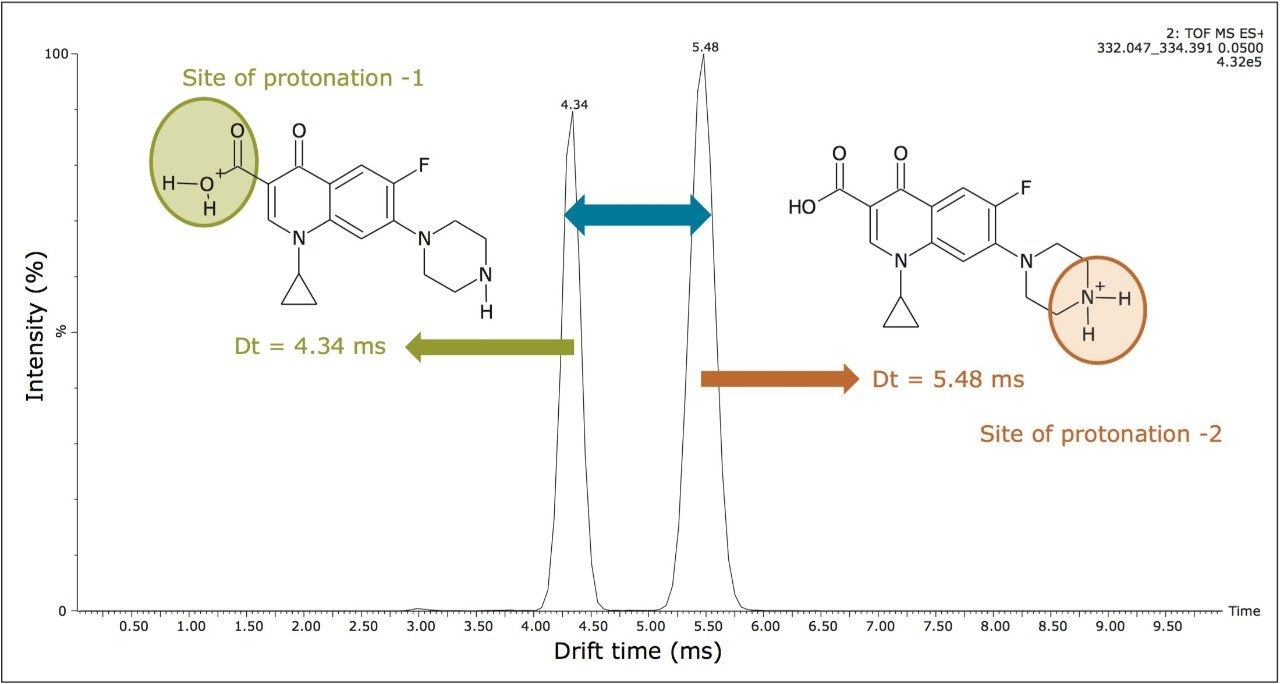
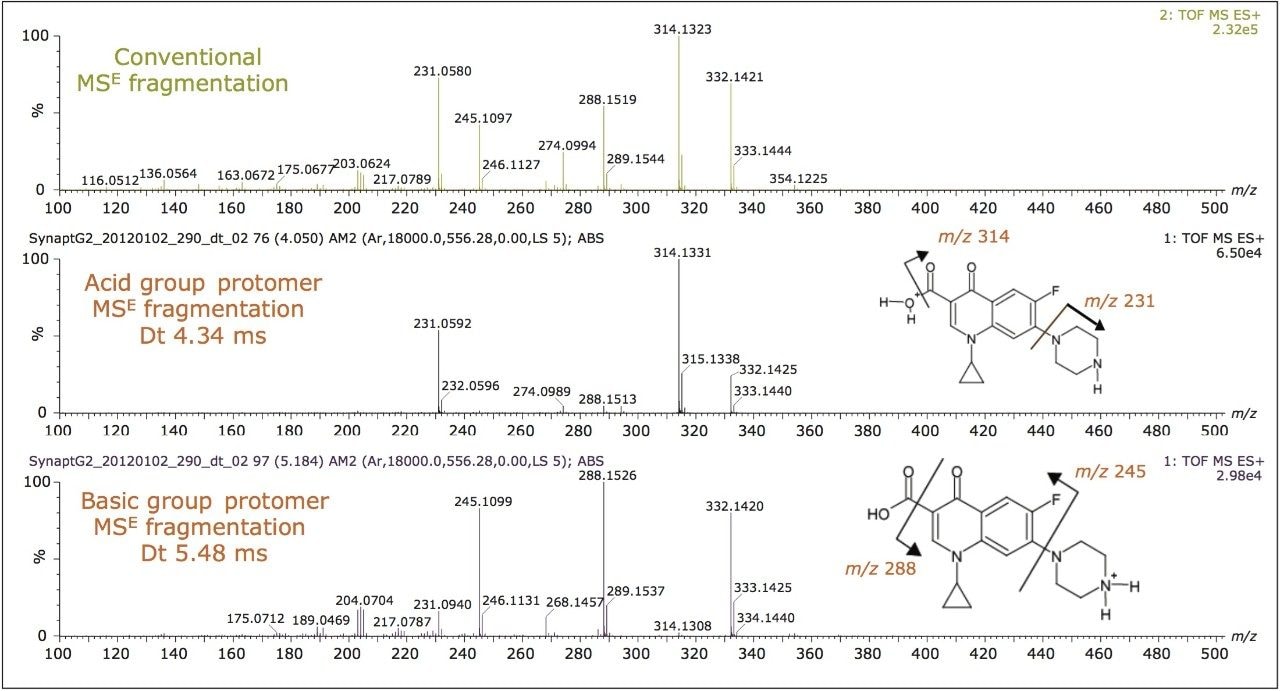
Each protomer has, therefore been treated as a single component, which has allowed the individual fragmentation spectra to be obtained (Figure 7). From the single component MSE fragmentation spectra, it was possible to determine that the two mobility separated species resulting from protonation takes place on both the acidic and basic groups for ciprofloxacin. The fragments at m/z 314 and m/z 231 (Figure 7) could only form from a species where ionization had taken place on the acidic group of ciprofloxacin. The fragments observed at m/z 288 and m/z 245 could only result if protonation had taken place on the basic group. The fragment m/z 231 is observed to form for both protomers. Further investigations have been performed regarding the fragmentation and will be presented in a separate study.
In Figure 4, the conventional MS spectrum generated in the UPLC HDMSE experiment is shown. At m/z 157 and m/z 166, two small, doubly charged ions of ciprofloxacin are apparent, indicating that ciprofloxacin did indeed also form a doubly charged species. Using ion mobility, it has been possible to separate and confirm that this small molecule does form a doubly charged species. The formation of the doubly charged species is dependent on the MS parameters used, especially the cone voltage. If the cone voltage is too high, the doubly charged species is not observed, only the [M+H]+.
The data confirm that further consideration should be given to method development and the means of analysis chosen, since the ratio and formation of the protomers varies with eluent flow rate, capillary voltage, cone voltage, and matrix. If MRM is the method of choice, consideration of the experimental conditions used and the specific transitions selected is imperative. The data illustrate that consistency in MRM transitions in inter- and intra-laboratory studies could easily be misinterpreted within and between different laboratories, explaining the challenges of achieving reproducible results for these types of compounds. Ion mobility is a valuable tool for method development to ensure method robustness and consistent results.
In addition to the scope of more specific and reliable method development, the drift times generated for the components analyzed can be used to produce an additional identification point. This application note described retention time, precursor ion accurate mass measurement, fragment ion accurate mass measurement, and two drift time values can be used as identification points for ciprofloxacin.
In addition to utilizing ion mobility to provide a new identification point, the orthogonal separation produced by ion mobility can be used for spectral cleanup. The plot of drift time versus retention time (Figure 8A) shows the ion intensity, represented by white pixels. The extent of the presence of matrix ions is shown by the expansive white color. The more intense analyte and matrix components are represented by the more vibrant white spots. However, it is difficult to see the low level target analytes due to the large amount of ion intensity produced by the matrix. In Figure 8B, the ciprofloxacin protomers have been extracted from the porcine matrix. Single component MS and fragmentation spectra can be produced from this clear, selective separation resulting from the use of ion mobility. The ratio of the acidic site protomer to the basic site protomer in the porcine matrix was determined to be 5:1 under these conditions. This is affected by capillary voltage, cone voltage, probe position, flow rate, and matrix. During infusion experiments, it was possible to vary the ratio of acidic/basic protomers and even change which was the most abundant protomer.
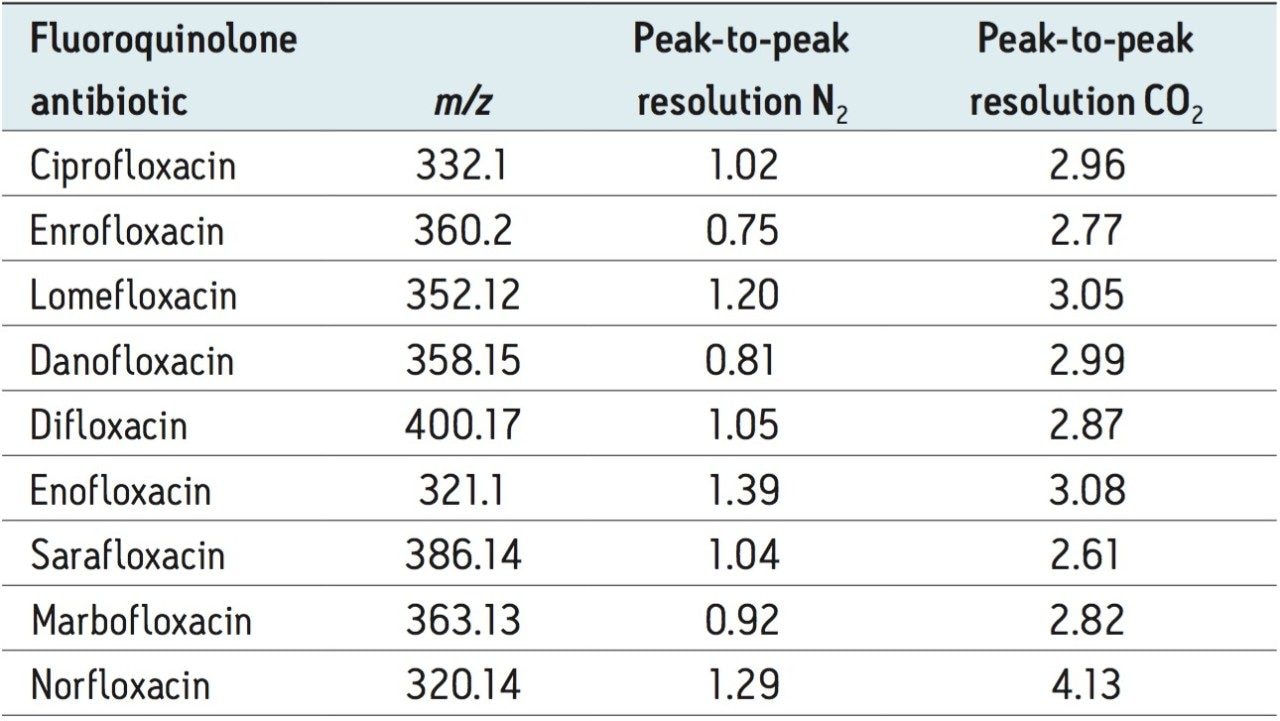
Figures 9 and 10 illustrate the protomer arrival time distributions for ciprofloxacin, norfloxacin, and difloxacin, as well as the calculated peak-to-peak resolution using N2 and CO2 drift gas, respectively. Although acceptable resolution was obtained using both gases, peak-to-peak values of Rs > 1.5 are considered to be fully resolved. Previous studies have considered the use of alternate drift gases to increase the ion mobility resolution.10-14
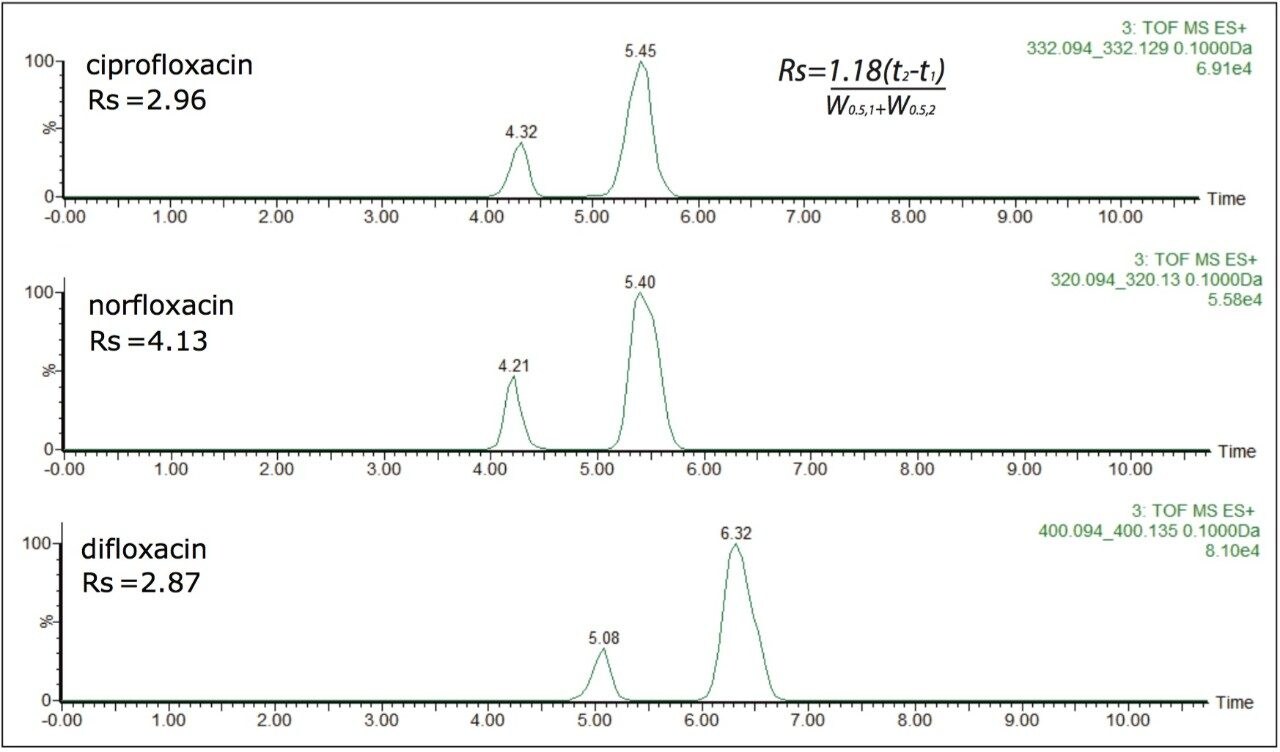

When performing IMS, the ion separation occurring in the travelling wave ion mobility (TWIM) drift cell is determined by the charge state, mass, shape, drift gas polarizability, as well as interaction between ion and neutral gas molecules. Increasing the polarizability of the drift gas increased the separation power (peak capacity) of TWIM in this application. As a result, the peak-to-peak resolution could be improved with consideration of the polarizability of the drift gas used, in this case CO2. The summary of the peak-to-peak resolution (Rs=1.18(ta-tb)/W0.5,a+W0.5,b) obtained using N2 or CO2 is shown in Table 1 (where Rs is the calculated resolution, the peak widths of peak A and B at half height are W0.5,a and W0.5,b respectively, and ta and tb are the respective drift times of peaks a and b). It can be seen that when using CO2 as the ion mobility drift gas, all the fluoroquinolone protomer pairs are fully resolved with peak-to-peak Rs between 2.61 and 4.13. The enhanced peak capacity using CO2 further improves the quality of the single component precursor ion and corresponding single-component fragmentation ion spectra obtained.
720004720, June 2013