The Waters ACQUITY UPLC System with fluorescence detection (FLR) combined with a Glycan Separation Technology (GST) Column provides superior resolution compared to HPLC systems. In this application note, a general guideline for researchers to optimize HILIC gradient conditions is shown. The focus is to develop a UPLC method with optimal resolution for 2-aminobenzamide (2-AB) labeled N-linked glycans released from human IgG.
The ACQUITY UPLC System used with Glycan Separation Technology Columns delivers the ability to achieve highly-resolved glycan separations in less time. This provides significant benefits to high-throughput glycosylation monitoring and profiling in biopharmaceutical drug development.
Monoclonal antibody (mAb) drug development has been the most active area in the biopharmaceutical industry in recent years. One of the important aspects in recombinant mAb development is to profile glycosylation patterns. Since the glycans play key functions in biological activities, the glycosylation variances during protein production affect the pharmaceutical properties such as efficacy and elimination rate. One of many analytical approaches for glycan analysis is hydrophilic interaction chromatography (HILIC) with fluorescence detection. It provides high sensitivity, good reproducibility, and the ability to separate complex glycan mixtures.
The Waters ACQUITY UPLC System with fluorescence detection (FLR) combined with a Glycan Separation Technology (GST) Column provides superior resolution compared to HPLC systems. These glycan columns, packed with 1.7-μm amide sorbent, efficiently separate the fluorescent-labeled glycans in HILIC mode. Highly-resolved glycan separations, especially for positional isomers and coeluting minor peaks, now can be more accurately measured in UPLC/FLR.
In this application note, a general guideline for researchers to optimize HILIC gradient conditions is shown. The focus is to develop a UPLC method with optimal resolution for 2-aminobenzamide (2-AB) labeled N-linked glycans released from human IgG.
We demonstrate the capabilities of glycan columns to separate fluorescent-labeled glycans in HILIC mode, including the positional isomers and coeluting minor peaks previously unresolved by HPLC.
|
LC system: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH Glycan Column 2.1 x 150 mm, 1.7 μm |
|
Column temp.: |
40 °C* |
|
Flow rate: |
400 μL/min* |
|
Mobile phase A: |
100 mM ammonium formate, pH 4.5* |
|
Mobile phase B: |
Acetonitrile |
|
Gradient: |
72 to 62% B in 45 min* |
|
Weak wash: |
72% acetonitrile |
|
Strong wash: |
20% acetonitrile |
|
Injection: |
1.0 μL partial loop |
(*All other conditions are indicated in figure legends.)
|
Detector: |
ACQUITY UPLC Fluorescence Detector |
|
Excitation: |
330 nm |
|
Emission: |
420 nm |
|
Data rate: |
5 pts/sec |
|
PMT gain: |
1.00 |
|
Time constant: |
Normal |
2-AB labeled N-linked glycans released from human IgG (ProZyme, San Leandro, CA, U.S.) were used. In addition, 2-AB labeled mannose 5 and mannose 6 were spiked into the sample to make a more chromatographically challenging sample.
2-AB labeled glycan separation was performed using the HILIC method. For method optimization, the focused gradient with shallow gradient slope was used to effectively separate the region where the interested glycans were closely eluting. Other chromatographic settings such as flow rate, buffer concentrations, pH, and column temperature were also varied to maximize the peak resolution in the same region. The optimized HILIC condition used in Figure 1 was established based on the examination of various settings, as illustrated in Figures 2 through 6.
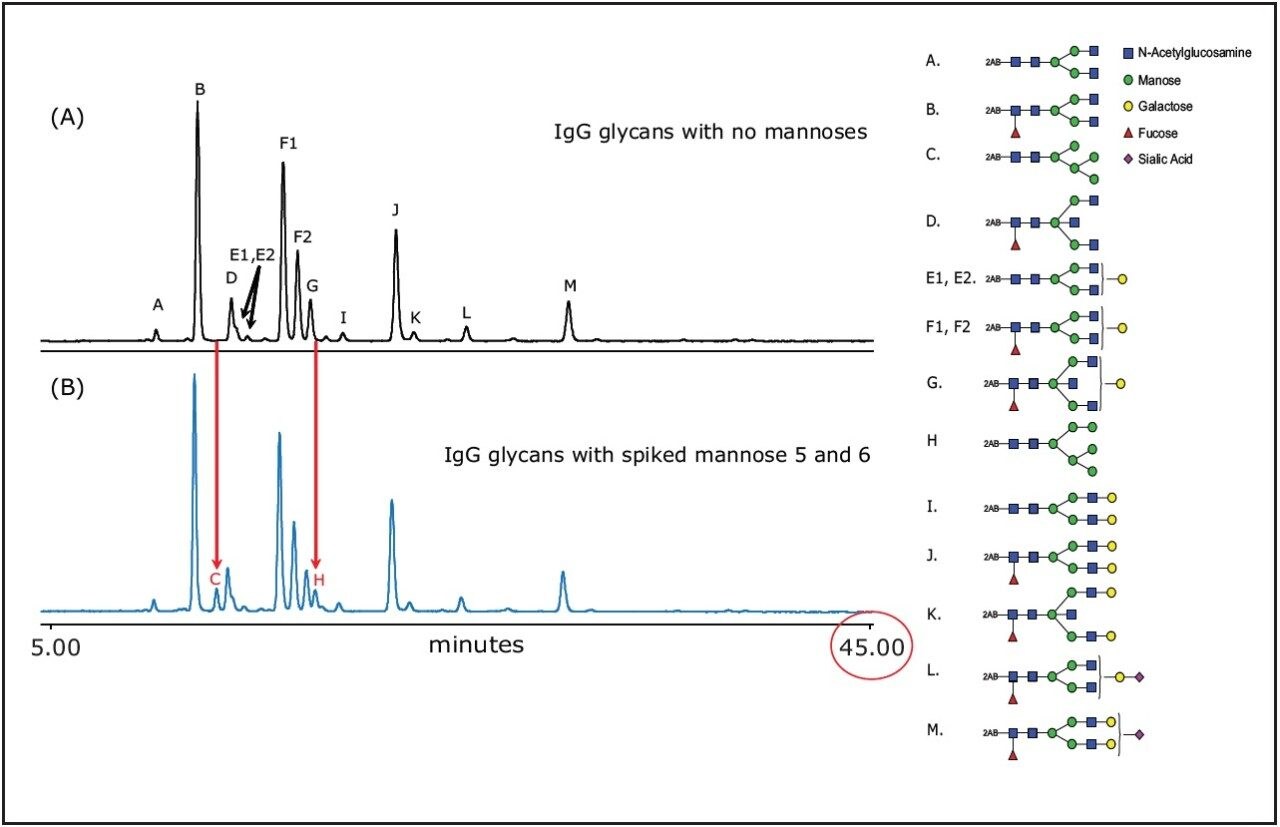
The complex mixture of 2-AB labeled glycans released from IgG was separated with superior resolution, as shown in Figure 1. All glycans were resolved including isomers E1/E2 and F1/F2. The gradient shown in all figures was run in 45 minutes and the entire run was completed in 1 hour using a 2.1 x 150 mm column. When the glycan HILIC method is transferred from a 3.0-μm HPLC to a 1.7-μm UPLC column, improved resolution in shorter run time can be achieved with UPLC system.
We demonstrate in Figure 1B that mannose 5 (peak C) and mannose 6 (peak H) can be successfully separated from their neighboring peaks, which often coelute.
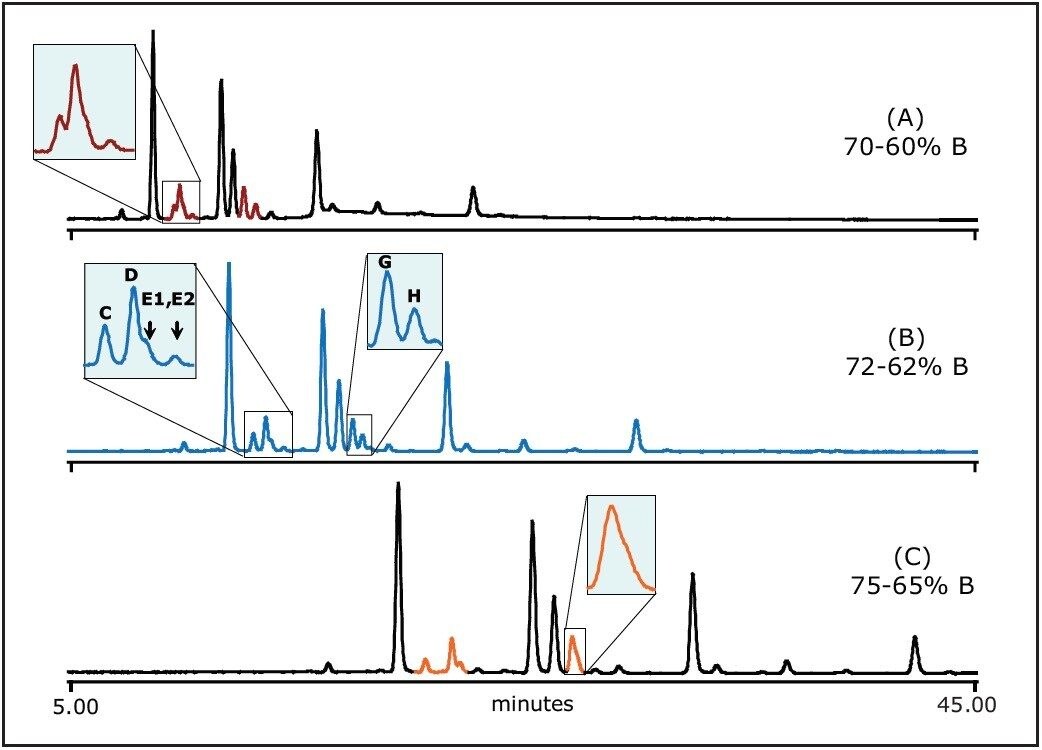
The impact of initial gradient strength on the separation is illustrated in Figure 2. The best separation was achieved at gradient condition 72% to 62% B. The resolutions in highlighted regions were changed positively and/or negatively. For example, the boxed region from A to B shows improved resolution; however the second boxed region from B to C shows the decreased resolution.
The gradient slope was set to 10% acetonitrile change in 45 min in all experiments in Figure 2 (0.22% B/min) to eliminate the gradient slope effect as a variable.
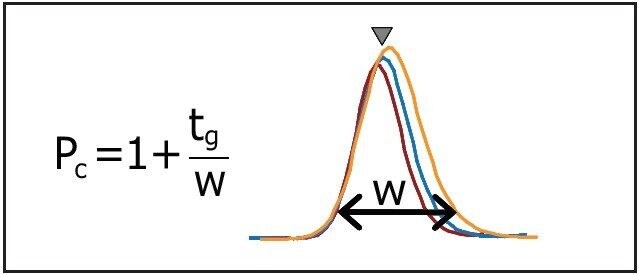
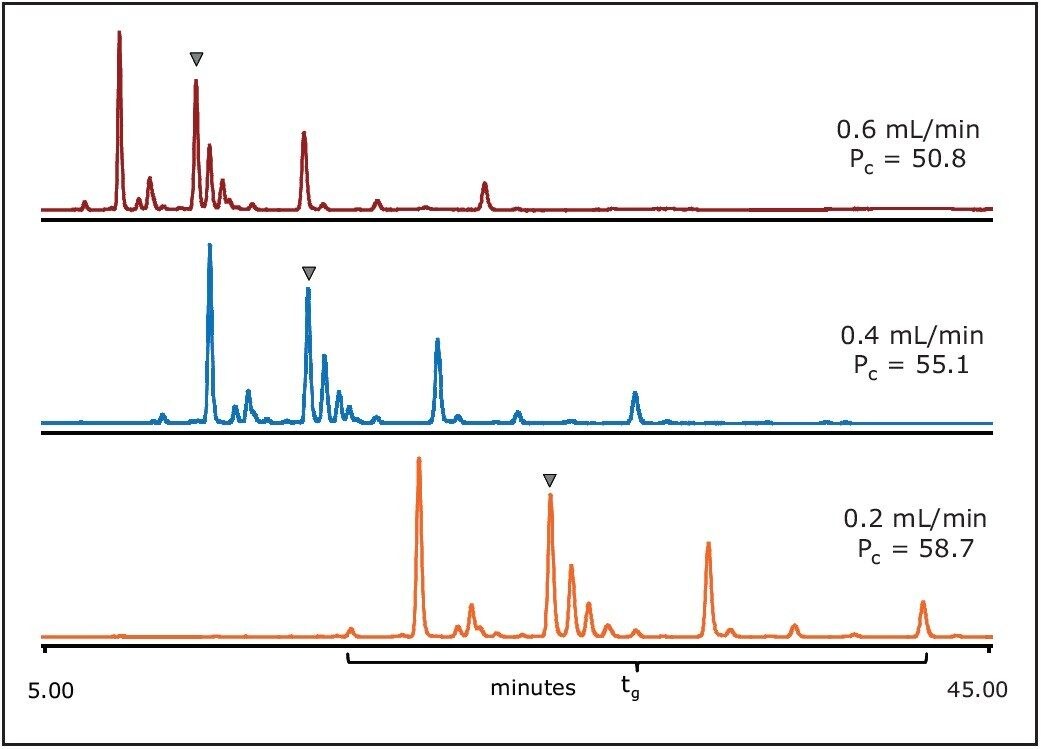
The separations in Figure 3 show the effect of the flow rate. Changing the flow rate influences the peak capacity as well as the resolution due to the contribution of the gradient slope change. When the flow rate increases from 0.2 to 0.6 mL/min the peak widths (w) become narrower; however, the effective separation window (tg) is simultaneously reduced (see Figure 3). Therefore, the impact of flow rate on peak capacity (defined as Pc = 1+tg/w) was moderate.
The flow rate 0.4 mL/min was chosen because it showed minor improvements in resolution. These gradient conditions were selected for further separation as described in the next section.
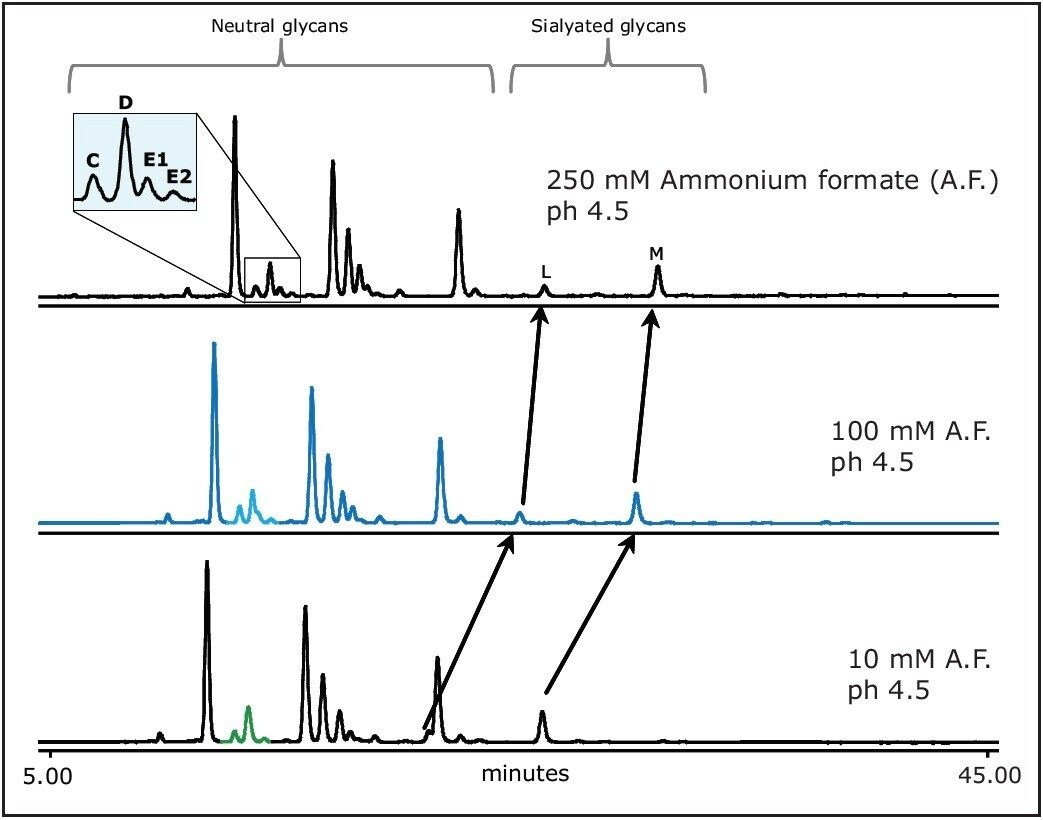
The buffer concentration affects the resolution and the selectivity, as shown in Figure 4. When elevating the ammonium formate concentration, the retention time of charged sialylated glycans is affected more significantly than the retention of neutral glycans. With mobile phase containing 250 mM ammonium formate we observed complete resolution of E1 from D, which was very difficult to achieve by varying other chromatographic conditions.
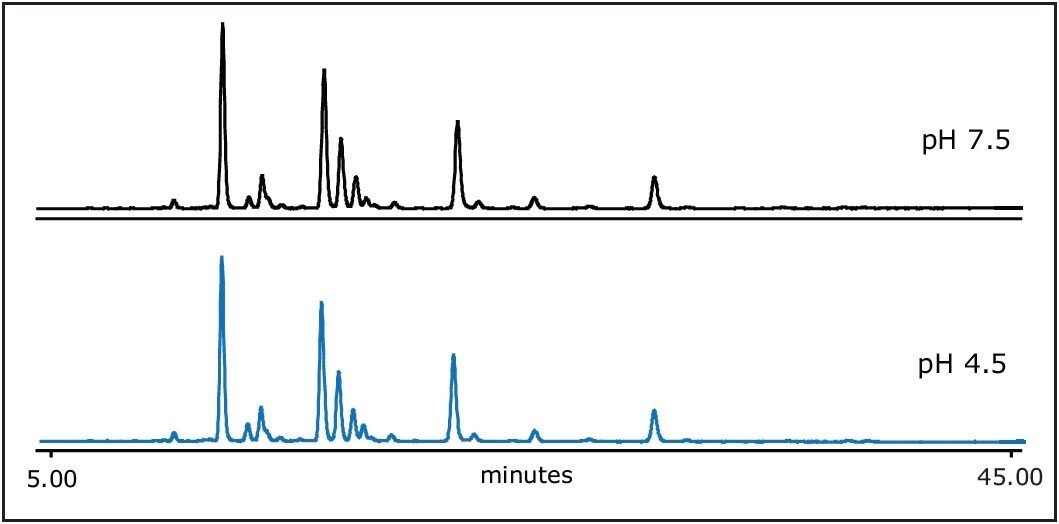
As shown in Figure 5, there is no significant change in separation between pH 4.5 and pH 7.5 in mobile phase A (100 mM ammonium formate). The mobile phase pH was adjusted by titrating ammonium with formic acid. However, the low pH in mobile phase will contribute to the retentivity shift of sialylated glycans (figure not shown).
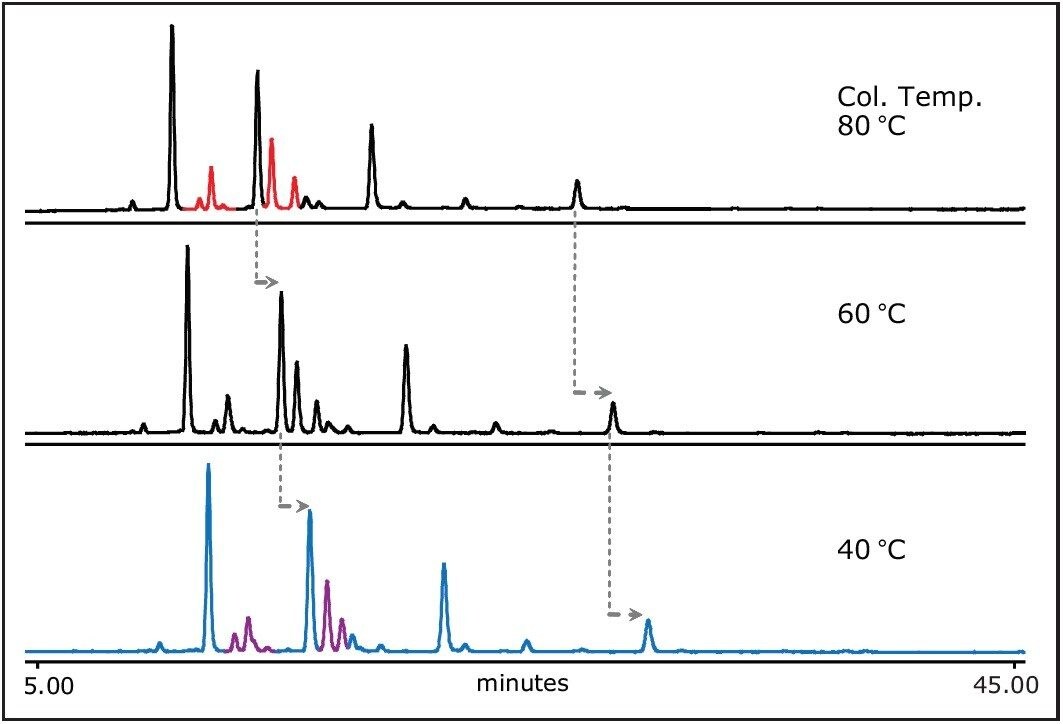
The column temperature affects the analyte retentively; all glycans shifted to earlier retention times with increased column temperatures as expected by chromatographic theory. The changes in highlighted regions marked in red and purple were also observed as a result of column temperature. Since the resolution is important for separating complex glycan mixture, the column temperature can be another parameter to be optimized (Figure 6).
720003238, February 2010