Analysis of Organic Carbonates in Lithium-Ion Battery Electrolyte by High-Performance Liquid Chromatography (HPLC) with Mass Detection (MS)
Margaret Maziarz, Stephanie Harden, Paul Rainville
Waters Corporation, United States
Published on January 15, 2026
Abstract
Lithium-ion battery electrolyte is a solution composed of a lithium salt dissolved in organic carbonates. The degradation of these carbonates is a key factor affecting battery efficiency and performance. This application note presents an HPLC-MS method for the analysis of common organic carbonates and carbonate-based aging products in electrolyte of the lithium-ion battery. The five carbonate solvents selected for the study included ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) and diethyl carbonate (DEC). Two aging products were tested, including dimethyl 2,5-dioxahexanedioate (DMDOHC) and diethyl 2,5-dioxahexanedioate (DEDOHC). Chromatographic separation was performed on an XSelect™ Premier HSS PFP Column, operated under reversed-phase conditions, with analysis carried out on an Arc™ Premier System coupled to an ACQUITY™ QDa™ II Mass Detector. The quantification limits for the carbonate solvents and aging products achieved using positive electrospray ionization and selected ion recording (SIR) acquisition mode ranged from 0.05 to 1 ppm and 0.00075 to 0.0025 ppm, respectively. Additionally, the analysis of lithium hexafluorophosphate (LiPF6)-based electrolyte solution was demonstrated.
Benefits
- Sensitive method for low-level detection and quantification of carbonate solvents (0.05–1 ppm) and carbonate-based aging products (0.00075–0.0025 ppm) in lithium-ion battery electrolyte solutions using the ACQUITY QDa II Mass Detector with SIR acquisition mode.
- Robust chromatographic separation using the XSelect Premier HSS PFP Column under 7 minutes analysis time, operated under reversed-phase conditions.
Introduction
The electrolyte is one of the main components in the lithium-ion battery.1,2 It is a conductive solution that transports lithium ions from the cathode to the anode during the charging process. During discharge, the lithium ions flow from the anode to the cathode. The electrolyte solution is composed of lithium salt dissolved in a mixture of organic solvents.1,2 The organic solvents are typically a mixture of cyclic and linear carbonates, including EC, PC, DMC, EMC, and DEC.2 Lithium hexafluorophosphate (LiPF₆) is commonly used as the conducting lithium salt,2 providing the necessary ionic conductivity for efficient battery operation.
The LiPF6 salt in carbonate-based systems can exhibit chemical and thermal instability, which can impact the performance of the lithium-ion batteries.2-4 Electrolyte solutions can also degrade, forming aging products including DMDOHC and DEDOHC.2 Various techniques are employed to investigate quality and properties of the electrolytes. Techniques such as scanning calorimetry (DSC) and thermogravimetric analysis (TGA) offer a comprehensive analysis of an electrolyte’s thermal properties, while rheology measures viscosity of the conductivity solutions.1 Additionally, chromatographic methods are used to measure the quantity of carbonate solvents in electrolyte solutions.2-4 Gas chromatography mass spectrometry (GC/MS) and liquid chromatography mass spectrometry (LC/MS) are common techniques used to investigate carbonate-based electrolytes.3
In this application note, an HPLC method with mass detection was developed for the analysis of five common carbonate solvents and two carbonate-based aging products (Table 1). The method employed an ACQUITY QDa II Mass Detector coupled with an Arc Premier System. Method performance characteristics including limit of detection (LOD), limit of quantification (LOQ), linearity and system suitability were demonstrated. Additionally, the developed method was applied for the analysis of LiPF6-based electrolyte solution.
Experimental
Materials
Methanol (Optima LC/MS) was purchased from Fisher Chemicals (catalog number A456-4). Formic acid (LC/MS) was purchased from Fisher Chemicals (catalog number 85178).
Organic carbonate solutions, carbonate-based aging products, and battery grade lithium hexafluorophosphate electrolyte were obtained from Sigma-Aldrich.
Sample Description
Standard Solutions
Individual stock solutions containing each carbonate solution and carbonate-based aging products were prepared at 10,000 ppm in methanol and used to make a mixture standard solution. Mixture standard solution was serially diluted with methanol to prepare LOD, LOQ, and linearity solutions.
Concentration in ppm represents a volume of analytes to the volume of solution (v/v).
Lithium Hexafluorophosphate Sample
Commercially available LiPF6 salt electrolyte solution in EC/DMC/DEC=1:1:1 (v/v/v) at 1.0 M was diluted with methanol at 1/5000 (v/v).
Method Conditions
|
System: |
Arc Premier System, Column Manager with active pre-heating, PDA and the ACQUITY QDa II Mass Detector |
|
Column: |
XSelect Premier HSS PFP Column, 2.5 µm, 4.6 mm x 100 mm (Waters, p/n: 186010051) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
10 °C |
|
Flow rate: |
1.0 mL/min |
|
Injection volume: |
5.0 µL |
|
Vials: |
LC-MS Maximum Recovery Vials 2 mL volume, p/n: 600000670CV |
|
Mobile phase: |
A: 0.1% formic acid in water B: 0.1% formic acid in methanol |
|
Wash solvents: |
Purge/Sample Wash: 60:40 water/methanol Seal Wash: 90:10 water/acetonitrile |
|
Separation: |
Described in the gradient table below |
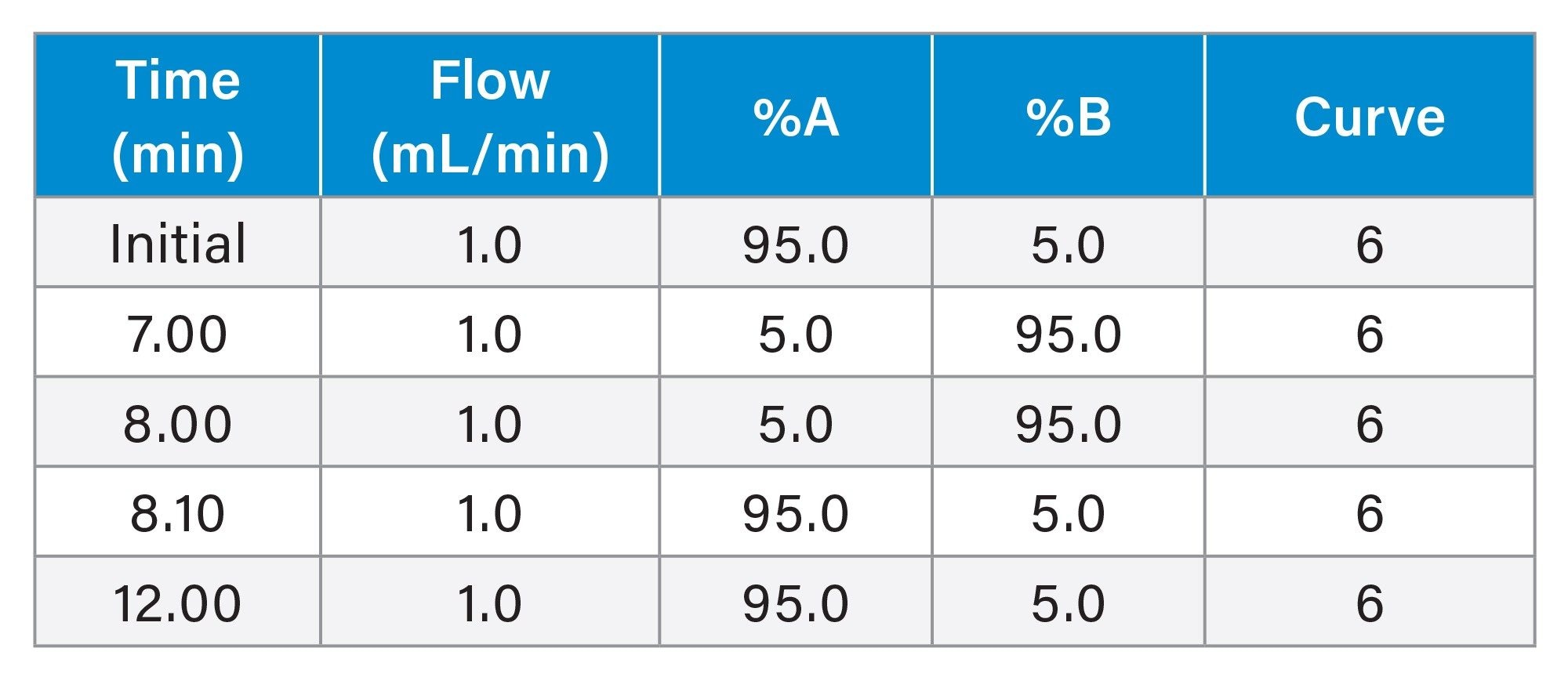
Gradient Table
MS Conditions
|
MS system: |
ACQUITY QDa II Mass Detector |
|
Ionization mode: |
Electrospray ionization in positive mode (ESI+) |
|
Acquisition: |
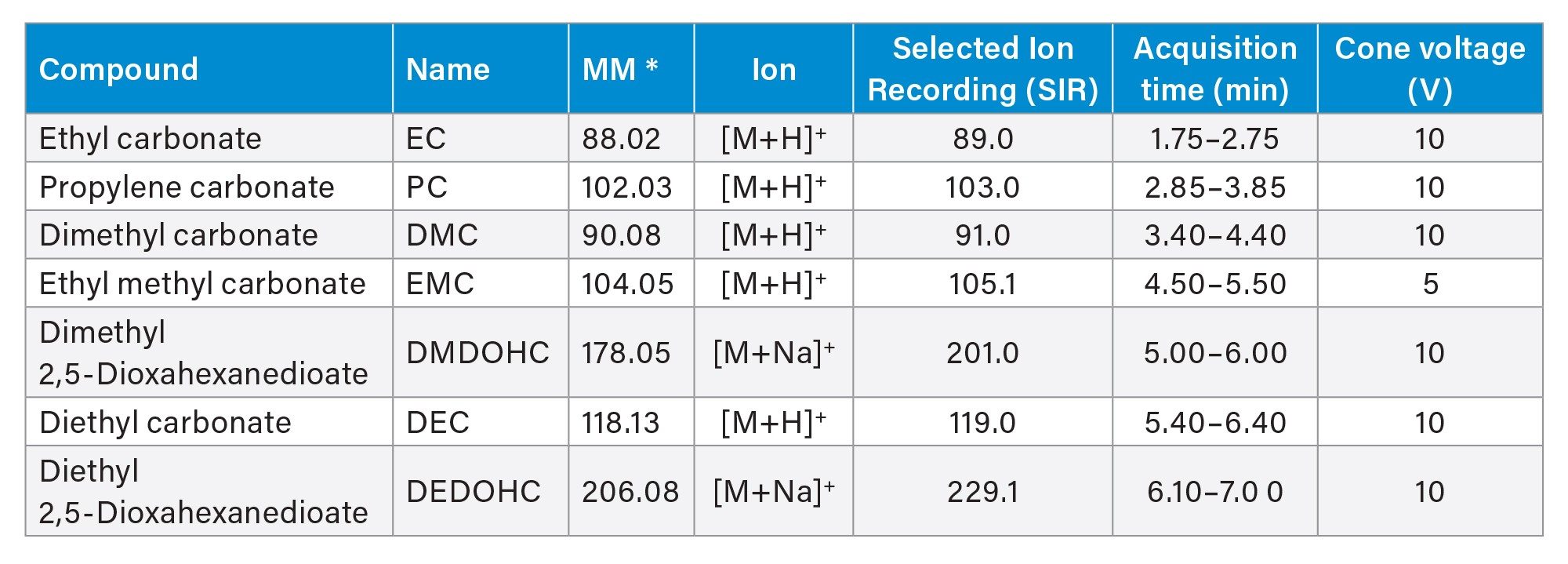
MS Scan: 35–300 Daltons SIR mode for quantitative analysis, as described in Table 1 |
|
Probe temperature: |
600 °C |
|
Capillary voltage: |
0.5 kV |
|
Data: |
Centroid |
Data Management
|
Chromatography data software (CDS): |
Empower™ 3.6.1 |
Results and Discussion
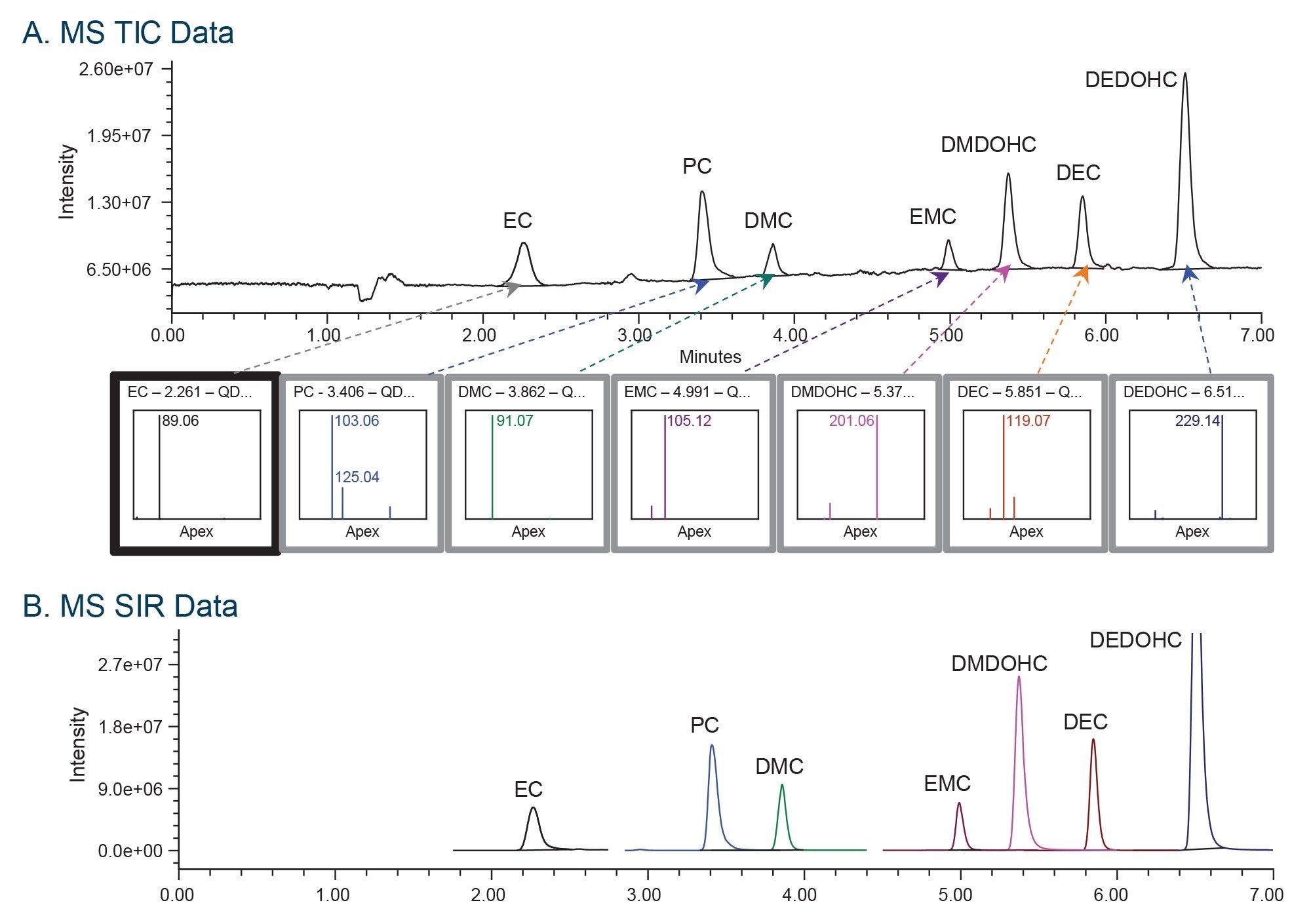
Chromatographic columns with a wide range of selectivity were explored during this study to ensure separation between carbonate solvents and carbonate-based aging products. The selected columns (XSelect Premier CSH™ C18, XSelect Premier CSH Phenyl Hexyl, XSelect Premier HSS T3, XSelect Premier HSS PFP) were tested with acetonitrile and methanol solvents. The study showed that XSelect Premier HSS PFP Column with methanol provided the best retentivity and separation for all analytes (Figure 1). The method successfully separated carbonate solutions and carbonate-based aging products in less than a 7-minute gradient time. The MS total ion chromatogram (TIC) data was collected across the mass range of 35–300 Da using electrospray ionization in positive (ESI+) mode (Figure 1A). The mass spectral data facilitated quick and accurate identification of the components. The carbonate solvents formed [M + H]+ molecular ions, while the selected aging products formed sodium adducts, [M + Na]+. These ions were measured using a selected ion recording (SIR) acquisition mode, which determines intensity for a single ion of interest (Figure 1B).
Optimization of Ionization Parameters
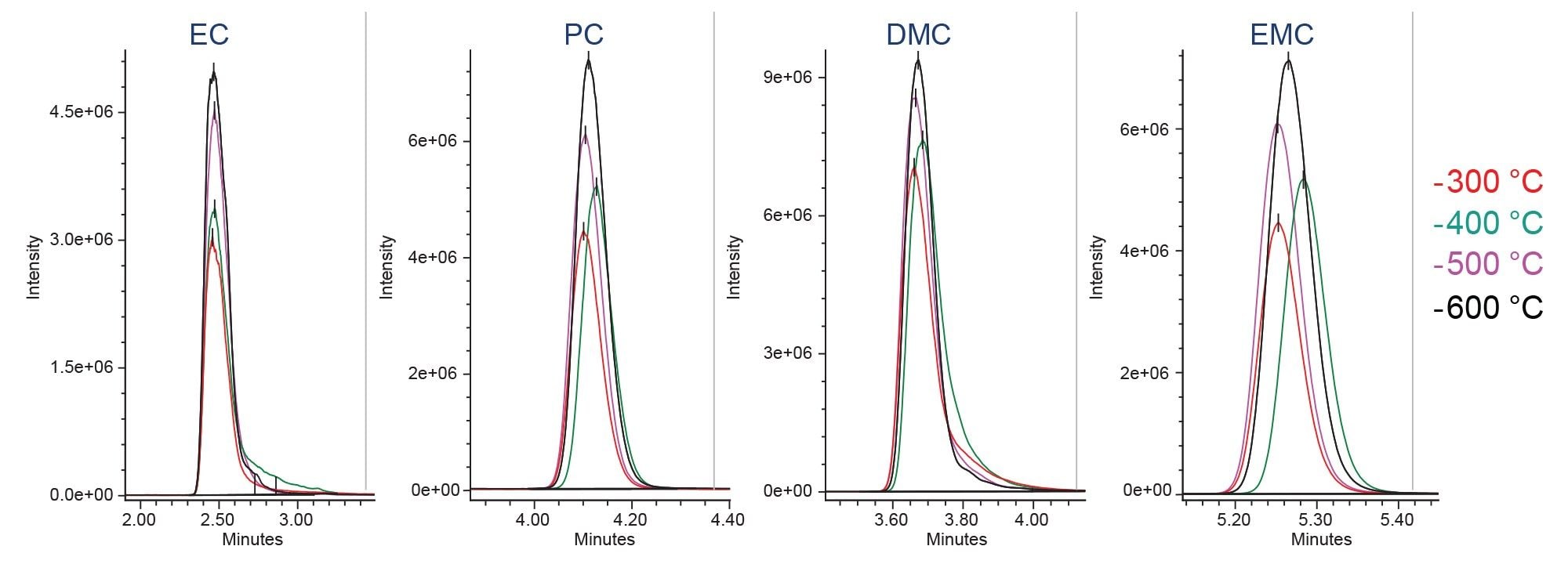
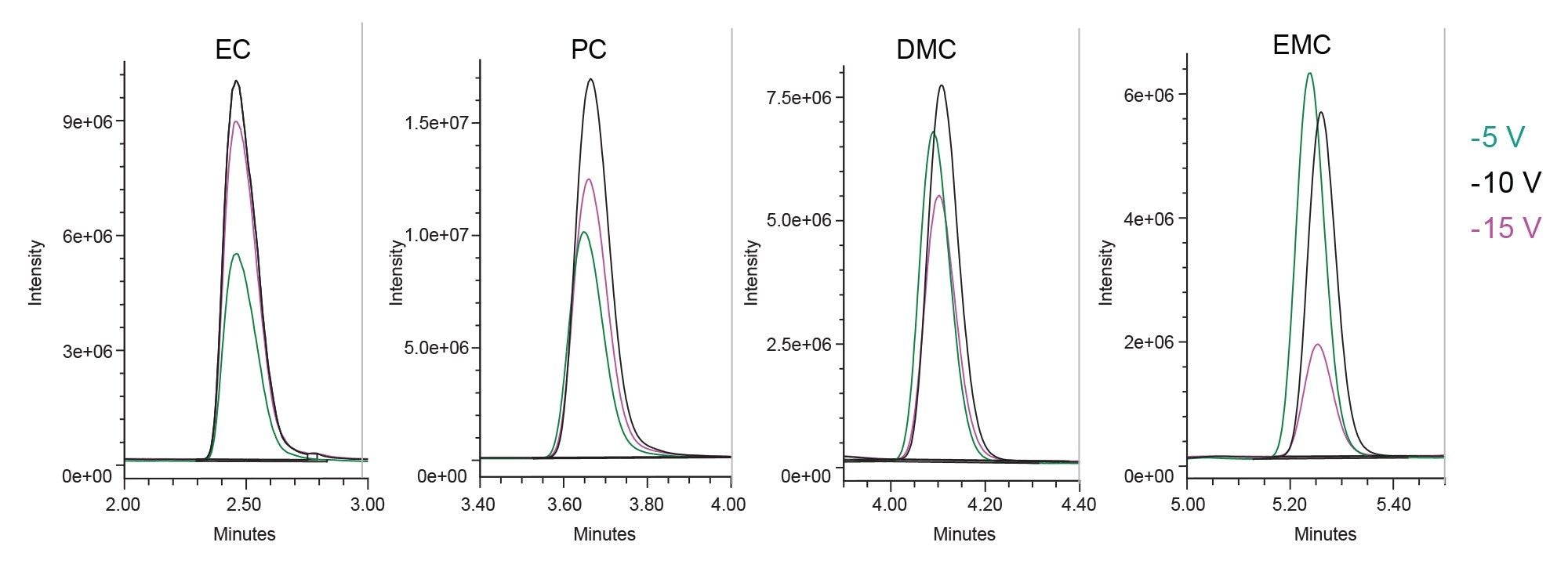
Ionization parameters, such as ionization mode, probe temperature and cone voltage are often optimized to enhance the MS signal and improve detection limits for target analytes. In this study, the impact of the ACQUITY QDa II Mass Detector ionization parameters on the sensitivity of carbonates solvents were evaluated. Probe temperature, cone voltage, and capillary voltage were systematically tested and examples of probe temperature and cone voltage optimization for EC, PC, DMC, and EMC are shown in Figures 2 and 3, with data acquired using SIR acquisition mode. A probe temperature of 600 °C produced the highest signal for carbonate solvents. Optimal cone voltages were 10 volts for EC, PC, DMC, and DMC, and 5 volts for EMC.
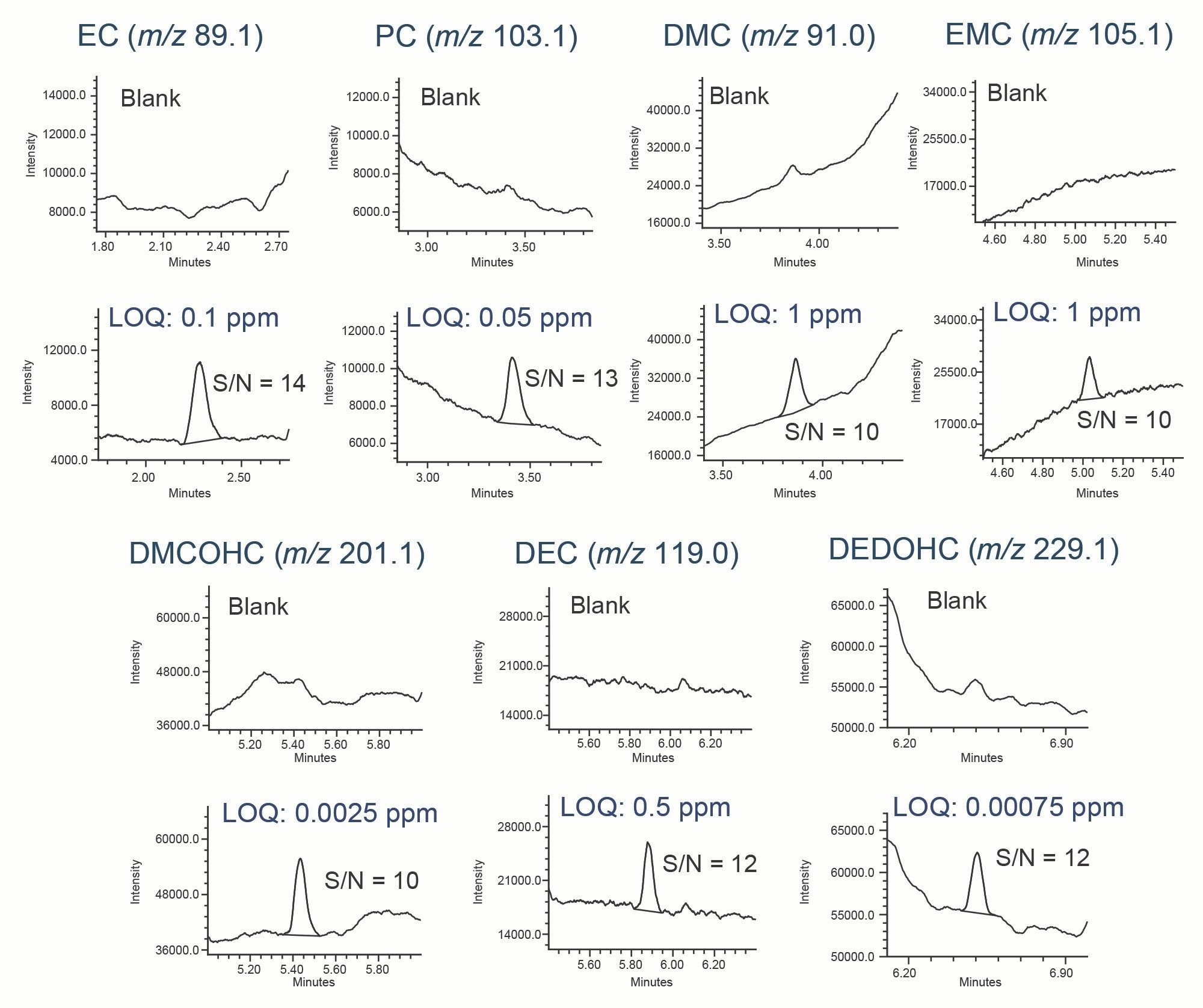
LOD and LOQ
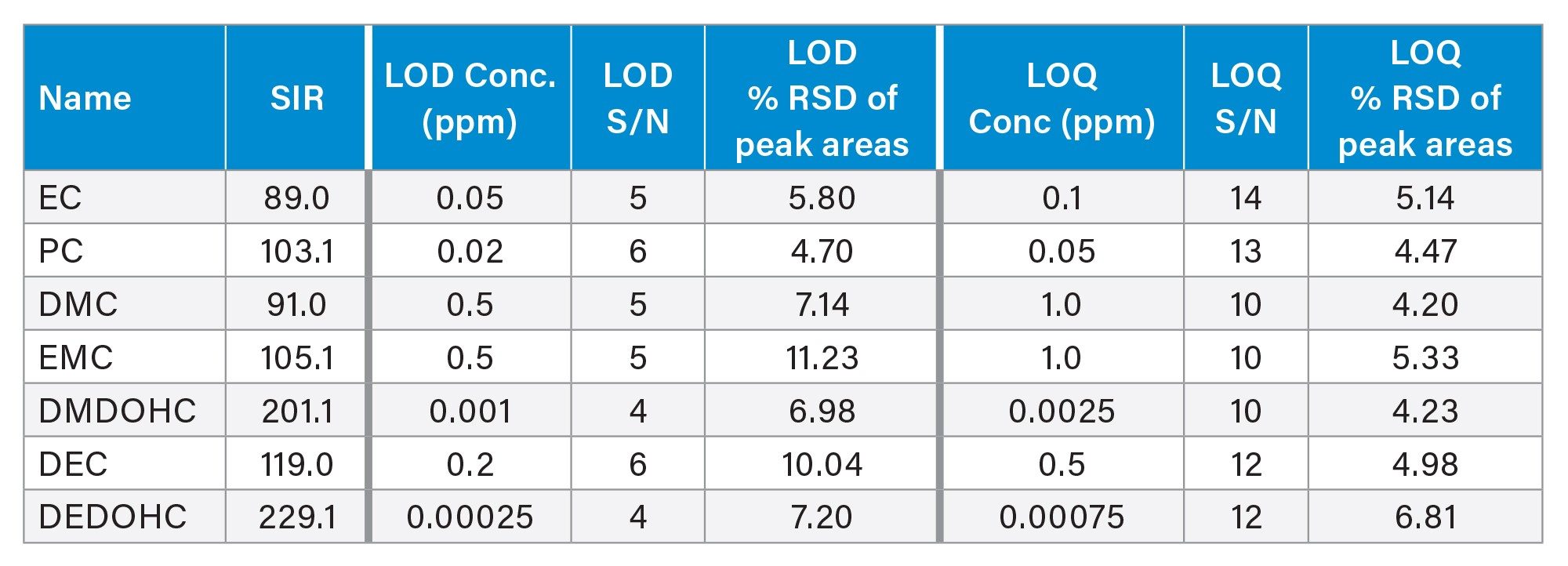
LOD and LOQ were determined using the signal-to-noise (S/N) criteria of 3:1 and 10:1, respectively. The S/N values were calculated using a noise centered on the peak region in the blank injection with the half height multiplier for noise region set to five, as recommended by the United States Pharmacopeia (USP).5 Representative chromatograms of the LOQ solutions prepared in methanol are shown in Figure 4. Data from six replicate injections was evaluated to establish and verify performance at the LOD and LOQ levels (Table 2). Excellent performance at the LOQ levels was achieved with the relative standard deviation (RSD) of the peak areas ≤ 6.81%. No internal standard was used in this work to correct for data variability.
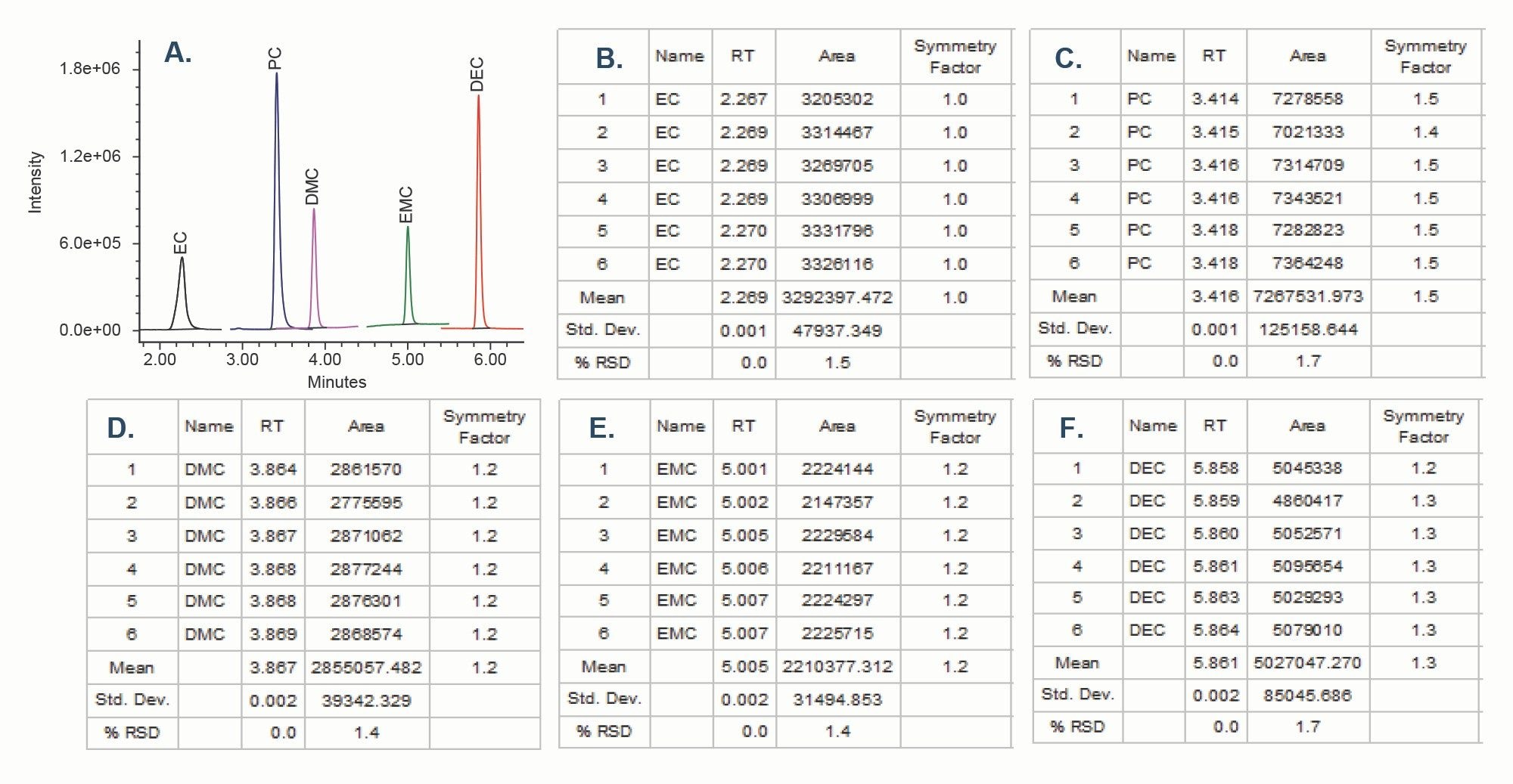
System Suitability
A system suitability test was performed to verify performance of the system and method. Data from 6 replicate injections of the standards solution containing carbonate solvents was used to determine %RSD of peak areas and retention times (Figure 5). The values for RSDs of peak areas were ≤ 1.7%.
Linearity
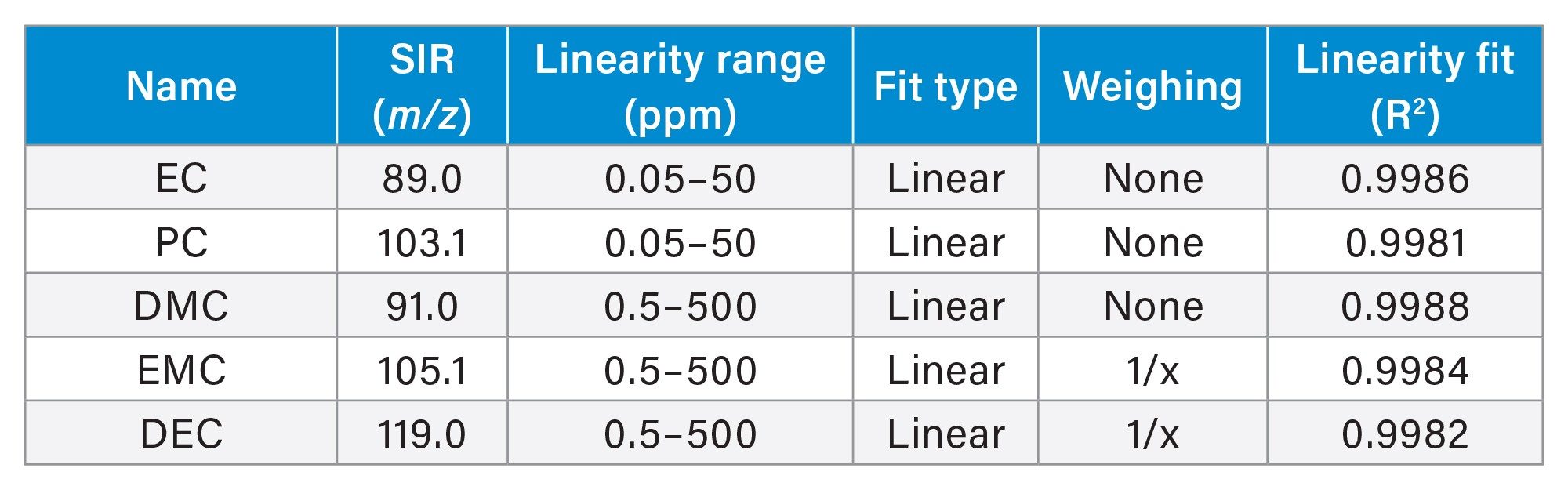
Method linearity for quantifying carbonate solvents over a concentration range of 0.05–50 ppm and 0.5–500 ppm for EP/PC and for DMC/EMC/DEC, respectively, was evaluated. The calibration curves exhibited a linear relationship between MS responses and concentration with correlation coefficients of R2 > 0.998 using a linear fit (Table 3).
Electrolyte Solution
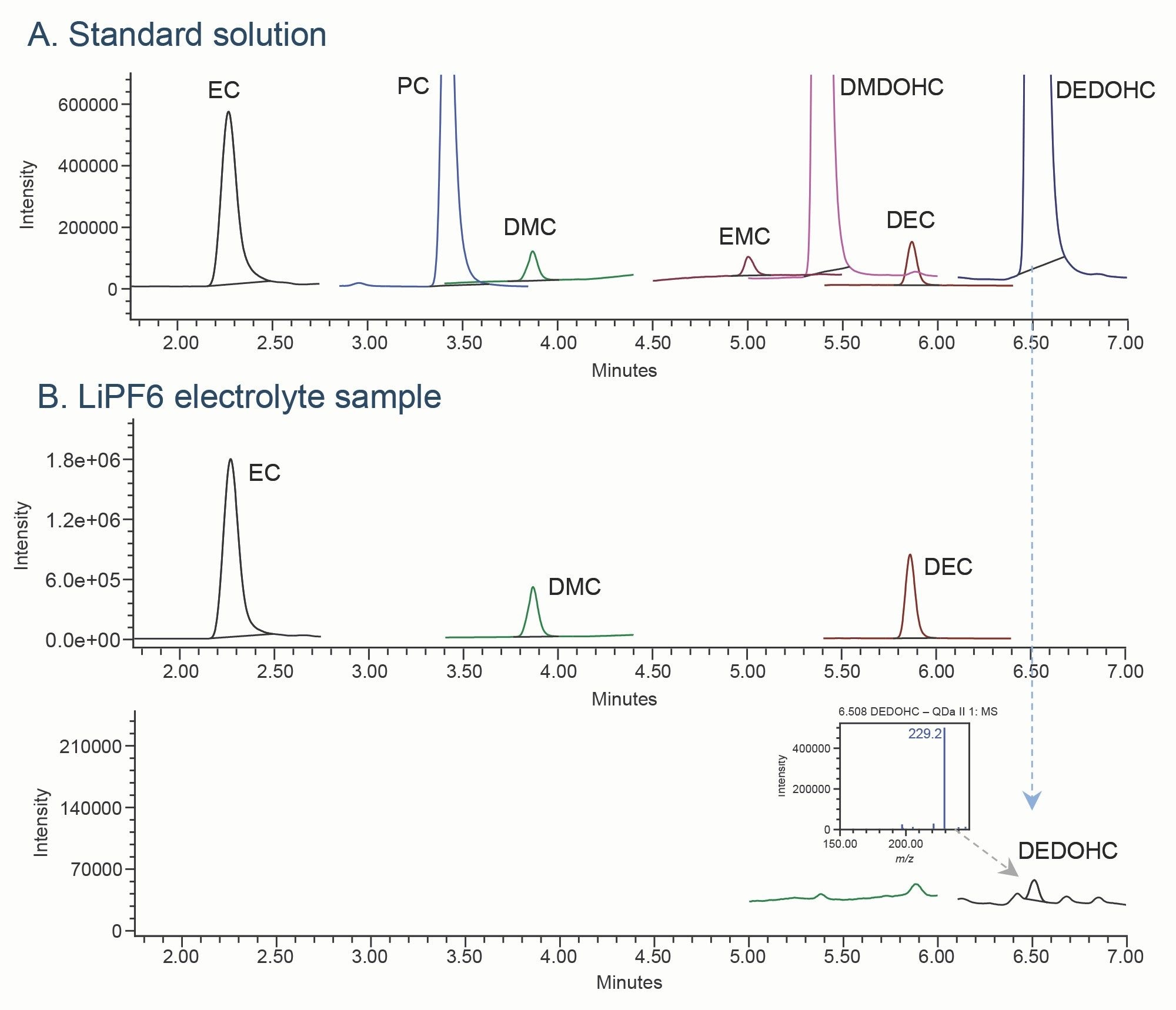
The developed method was applied for the analysis of commercially available electrolyte containing LiPF6 salt in EC/DMC/DEC = 1:1:1 (v/v/v) at a 1.0 M concentration. Sample was diluted at 1/5000 (v/v) in methanol. The analysis confirmed the presence of carbonate solvents EC, DMC, and DEC, as well as the trace-level of a carbonate aging product DEDOHC (Figure 6).
Conclusion
A sensitive and reliable HPLC-MS method using the Arc Premier System coupled with the ACQUITY QDa II Mass Detector was developed for the analysis of carbonate solvents and carbonate-based aging products in lithium-ion battery electrolyte. This reversed-phase LC method successfully separated all five carbonate solvents (EC, PC, DMC, EMC, DEC) and two aging products (DMDOHC/DEDOHC) on an XSelect Premier HSS PFP Column. Low-level quantification limits were achieved using SIR acquisition mode ranging from 0.05 to 1 ppm and 0.00075–0.0025 ppm for carbonate solvents and aging products, respectively. The method was demonstrated to be applicable for the analysis of lithium-ion battery electrolyte.
References
- Waters TA Instruments, Lithium-Ion Battery Material Testing.
- Schultz C, Vedder S, Streipert B, Winter M, Nowak S. Quantitative investigation of the decomposition of organic lithium-Ion Battery Electrolytes with LC-MS/MS. RSC Advances, 2017, 7:27853–28762.
- Bhalkikar A, Marin CM, Cheung CL, Method development for Separating Organic Carbonates by Ion-Moderated High-Performance Liquid Chromatography. Journal of Separation Science, 2016, 39: 4484–4491.
- Fang C, Tran TH, Zhao Y, Liu G. Electrolyte Decomposition and Solid Electrolyte Interphase Revealed by Mass Spectrometry. Electrochimica Acta, 2021, 399: 139362.
- USP General Chapter <621>, Chromatography. United States Pharmacopoeia USP-NF 2025 Issue 3, Official 01-Dec-2024.
720009211, January 2026