Application Brief
This is an Application Brief and does not contain a detailed Experimental section.
This is an Application Brief and does not contain a detailed Experimental section.
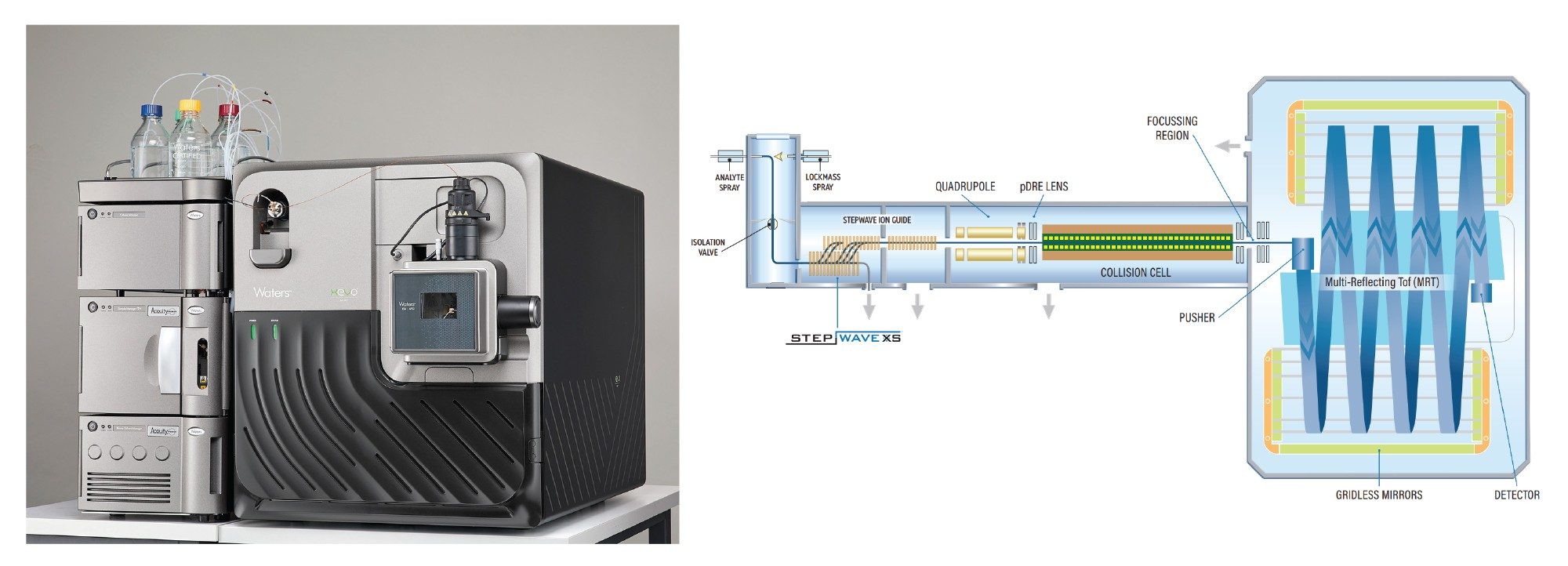
In this Technology Brief, we demonstrate the use of the ACQUITY™ Premier UPLC System coupled to the Xevo™ Multi-reflecting Time-of-Flight (MRT) Mass Spectrometer, for the analysis of mAb Subunit derived from the NIST Reference mAb standard. The Waters INTACT Mass™ App within waters_connect™ Software Platform was used to process the data, providing comprehensive insights into the product variants localized to each of the subunits. The results highlight the high sensitivity, high mass resolution and consistent sub ppm mass accuracy of the Xevo MRT MS platform, along with the capability for integrated data acquisition, processing, and review using the waters_connect informatics platform INTACT Mass Application.
Subunit based analysis of monoclonal antibodies has gained favor as an analytical tool for characterization and monitoring of product variation. Subunits typically require far simpler sample preparation prior to LC-MS analysis, compared to peptide mapping, and can reveal lower levels of product variation vs. Intact mAb analysis due to the division of the 150 kD mAb into roughly 25 kD subunits.
The Xevo MRT Mass Spectrometer System uses a novel detector geometry and instrument design to deliver exceptional sensitivity, dynamic range, mass resolution, and mass accuracy for mAb subunit analysis. This Q-Tof System incorporates a novel multi reflecting time-of-flight design to effectively extend the ion flight path, resulting in mass resolutions of up to 100K that are independent of scan rate, and remain consistent across a wide m/z acquisition region. The dual gain ADC detection system enables greater sensitivity and a wide dynamic range while delivering sub-ppm to low ppm mass accuracy for biomolecule analysis. This combination of high resolution, sensitivity and mass accuracy enables detailed protein characterization analyses that are crucial for understanding the structure, and function of biopharmaceuticals. The Xevo MRT Mass Spectrometer System can detect and characterize complex and lower intensity protein variants such as those arising from differential N-terminal processing, glycosylation and oxidation, all of which can significantly impact the efficacy, stability, and safety of biopharmaceuticals.
In this study, collected data were processed using the waters_connect INTACT Mass application, allowing researchers to confirm product identify, and profile variation on the individual mAb subunits. By providing these detailed insights into the molecular composition of biopharmaceutical products during early characterization studies, the Xevo MRT Mass Spectrometer System helps ensure consistency and stability of final product using this same approach during later development stages to ensure quality and consistency of biotherapeutic products as they are brought through commercialization.1,2
Waters mAb (NIST mAb) Subunit Standard, 25 µg in vial, Waters p/n: 186008927 was prepared by adding 100 µl Milli-Q water to resulting in a concentration of 250 ng/µl.
Ten replicate 250 ng injections of the Waters mAb Subunit Standard were acquired and processed using the waters_connect INTACT Mass App. Automated chromatographic peak selection and spectral deconvolution were employed for streamlined mass confirmation, impurity profiling, and purity assessments.
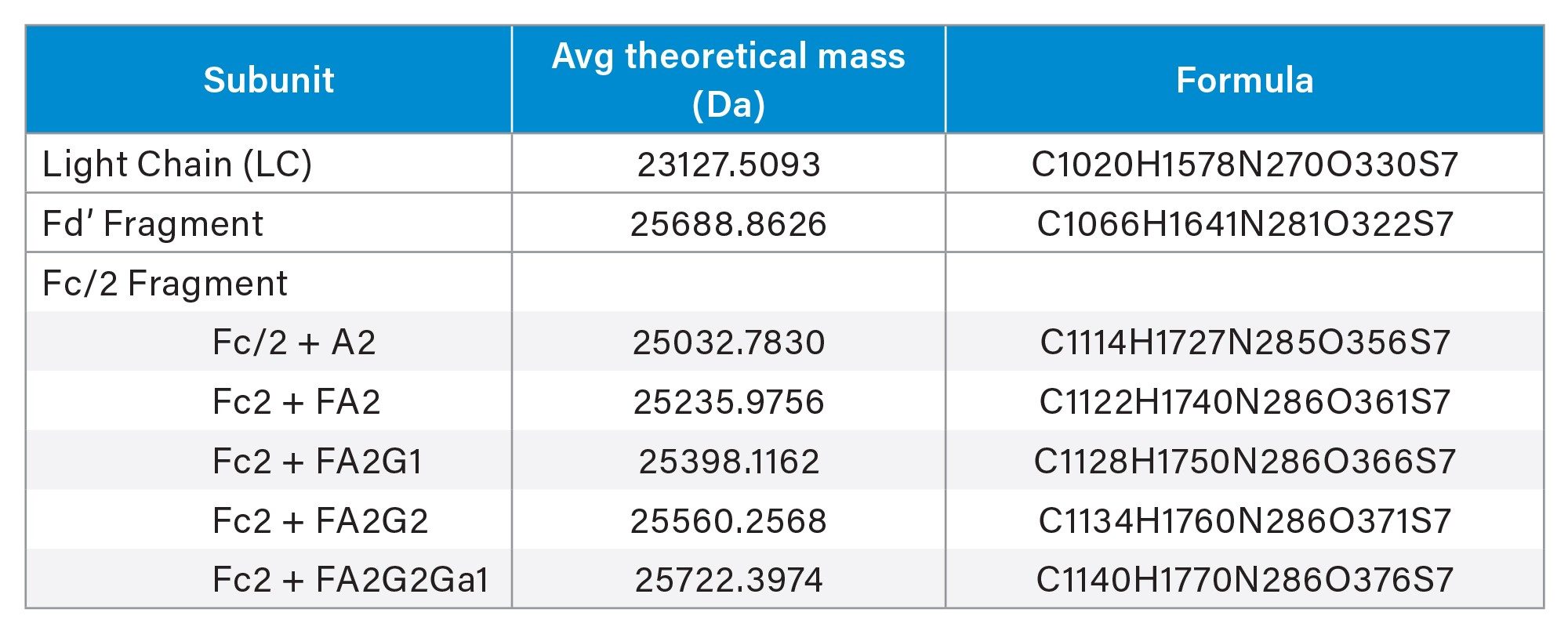
The theoretical mass of subunit species are displayed below.
|
LC system: |
ACQUITY Premier UPLC System (Binary) |
|
|
Detection: |
UV 280 nm |
|
|
Vials: |
TruView LCMS Certified 12 x 32 mm Screw Neck Vial, Total Recovery, with Cap and Preslit PTFE/Silicone Septum (p/n: 186005663CV) |
|
|
Column: |
ACQUITY Premier Protein BEH C4, 300 Å, 1.7 µm 2.1 mm x 50 mm (p/n: 186010326) |
|
|
Column temperature: |
60.0 °C |
|
|
Sample temperature: |
6.0 °C |
|
|
Injection volume: |
1 µL |
|
|
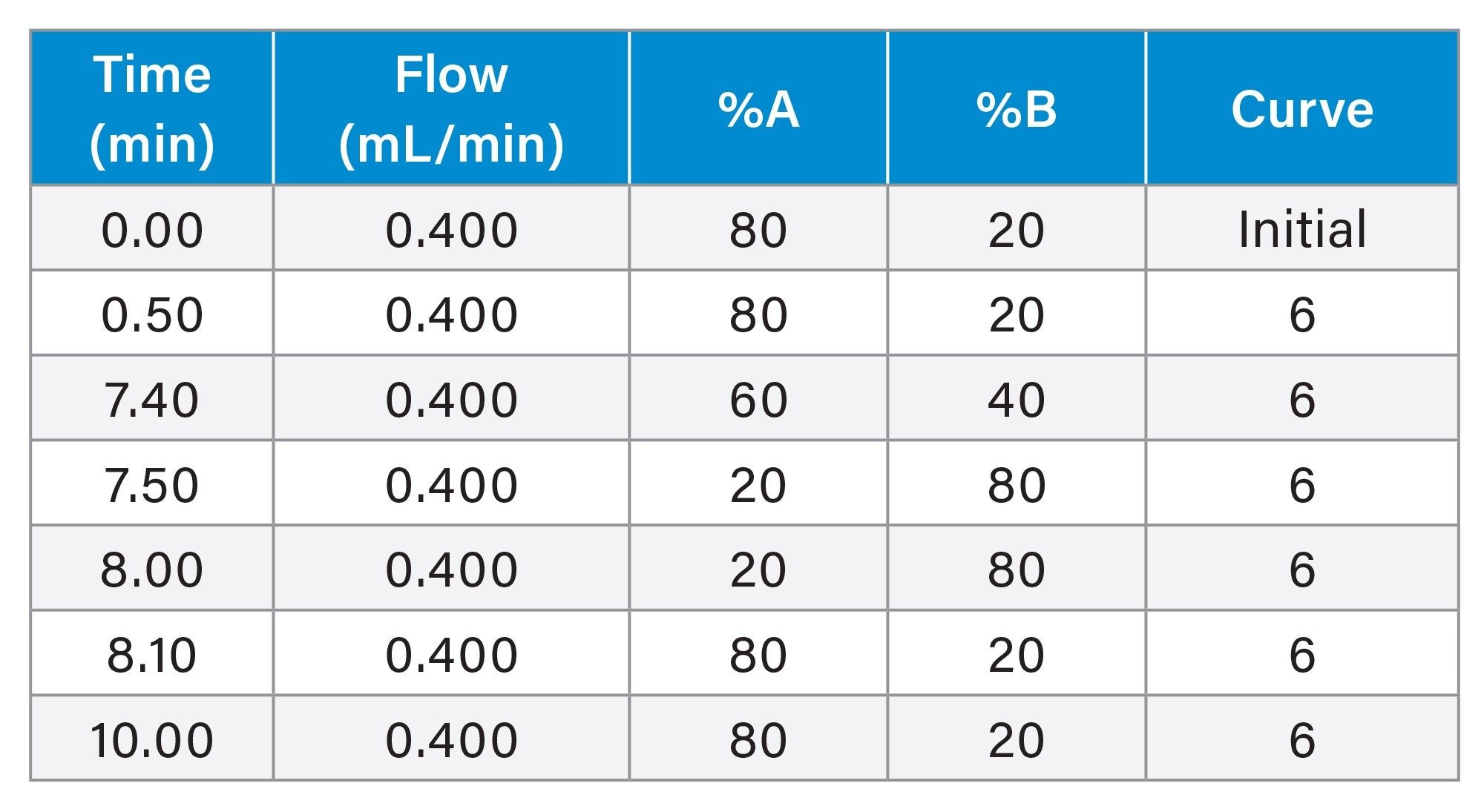
Flow rate: |
0.400 ml/min |
|
|
Mobile phase A: |
H2O 0.1% formic acid |
|
|
Mobile phase B: |
ACN 0.1% formic acid |
|
MS system: |
Xevo MRT Mass Spectrometer |
|
Mode: |
MS |
|
Mass range: |
400–4000 m/z |
|
Polarity: |
Positive |
|
Scan rate: |
1 Hz |
|
Cone voltage: |
40 V |
|
Cone gas: |
0 L/hr |
|
Source temperature: |
120 °C |
|
Desolvation temperature: |
500 °C |
|
Desolvation gas: |
800 L/hr |
|
Capillary voltage: |
3.00 kV |
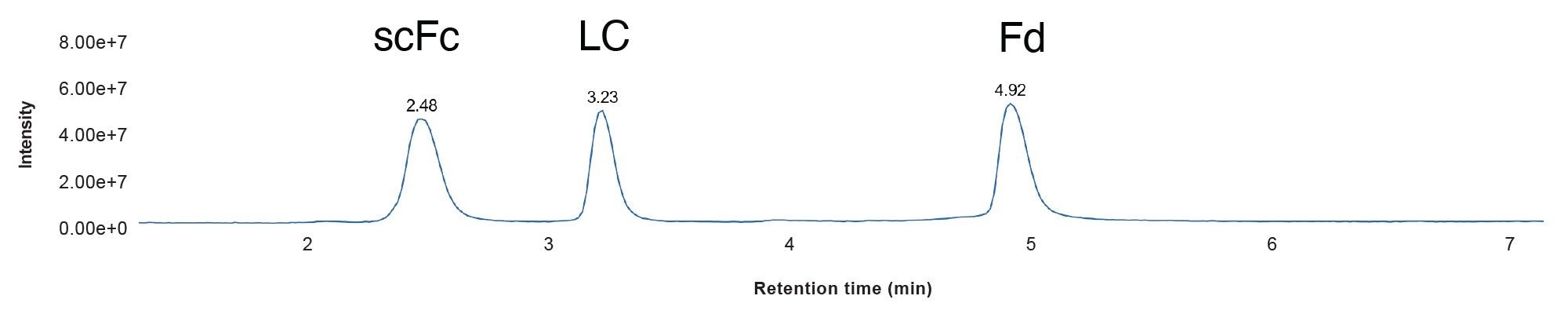
The mAb Subunit analysis provides several advantages over peptide mapping for monoclonal antibody (mAb) analysis. It features faster and simpler workflows with fewer sample preparation steps, enabling consistency of samples for analysis and enabling the simultaneous characterization of multiple attributes at the domain level, making it a valuable tool for antibody characterization and product quality monitoring. In this study, data on a Waters subunit standard derived from the NIST mAb Reference Standard was acquired on the ACQUITY Premier UPLC-Xevo MRT Mass Spectrometer System to assess overall workflow performance and report specific instrument capabilities for mass accuracy, mass resolution, and sensitivity. Figure 2 through 5 illustrate the chromatographic separation of the IdeS digested subunits (Figure 2), as well as the raw (Figure 3 and 4) and processed data (Figure 5), that convey system performance, and figures of merit for this routine mAb characterization approach.
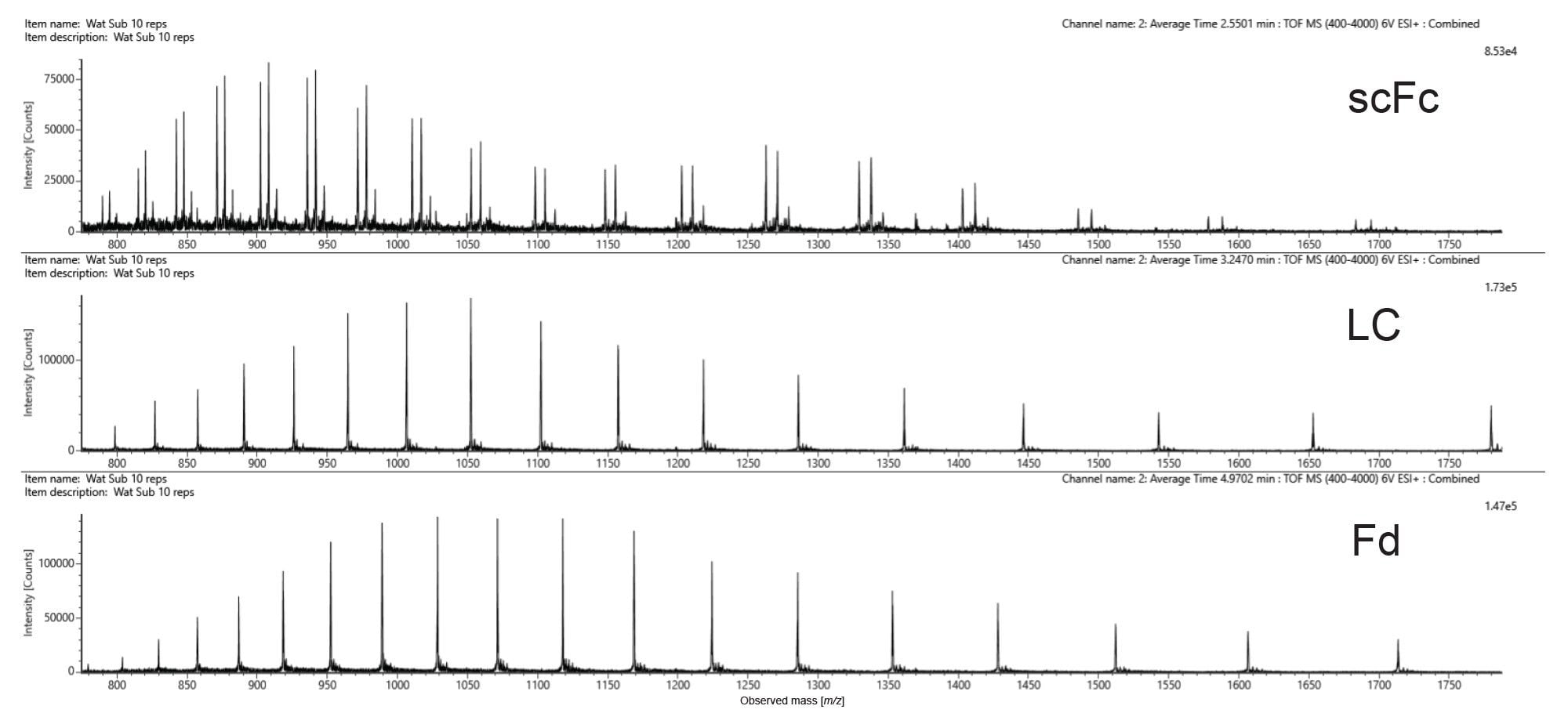
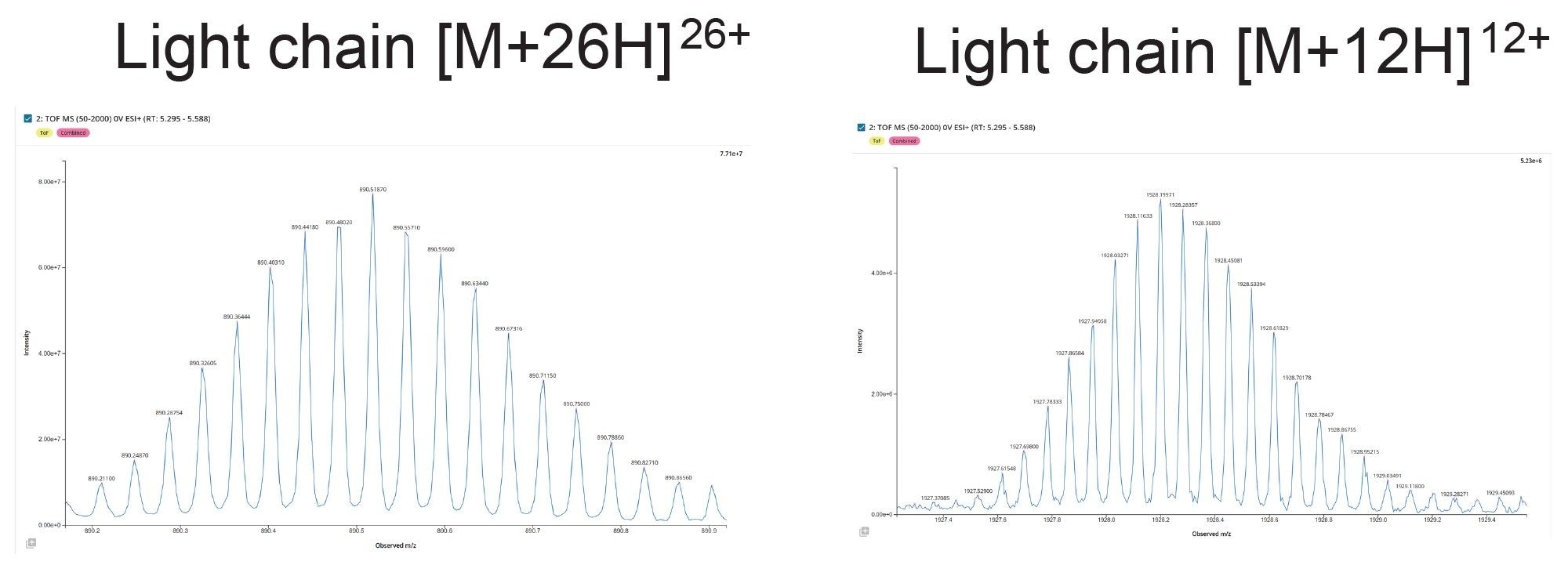
The waters_connect INTACT Mass App can be used to automate the process of mass confirmation and purity assessment for a wide range of biomolecule classes.
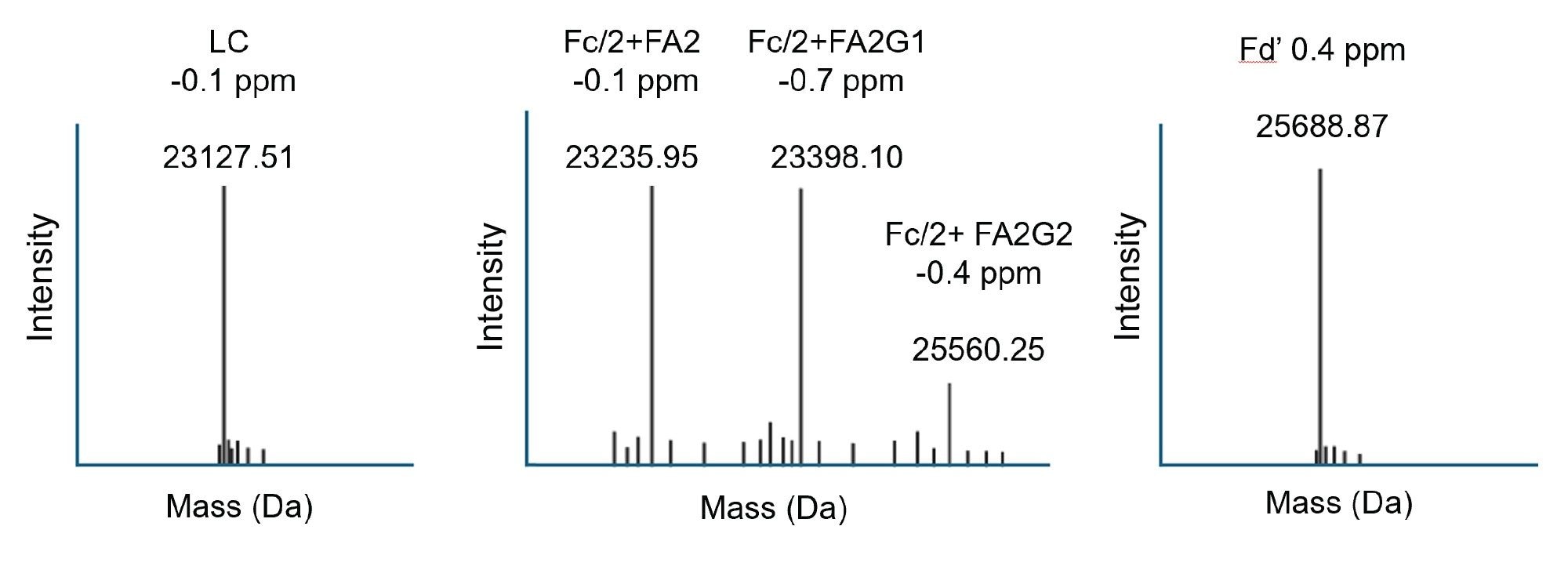
The output of automated INTACT Mass App LC-MS analysis of the IdeS digested NIST mAb subunits was produced using the BayesSpray deconvolution algorithm. Processed data (Figure 6) is displayed as it appears in the INTACT Mass App data review window. The Xevo MRT Mass Spectrometer based analysis enables the differentiation of closely spaced glycoform peaks, while the increased sensitivity allows for the detection of lower-abundance glycovariants with mass errors consistent across all glycovariants.
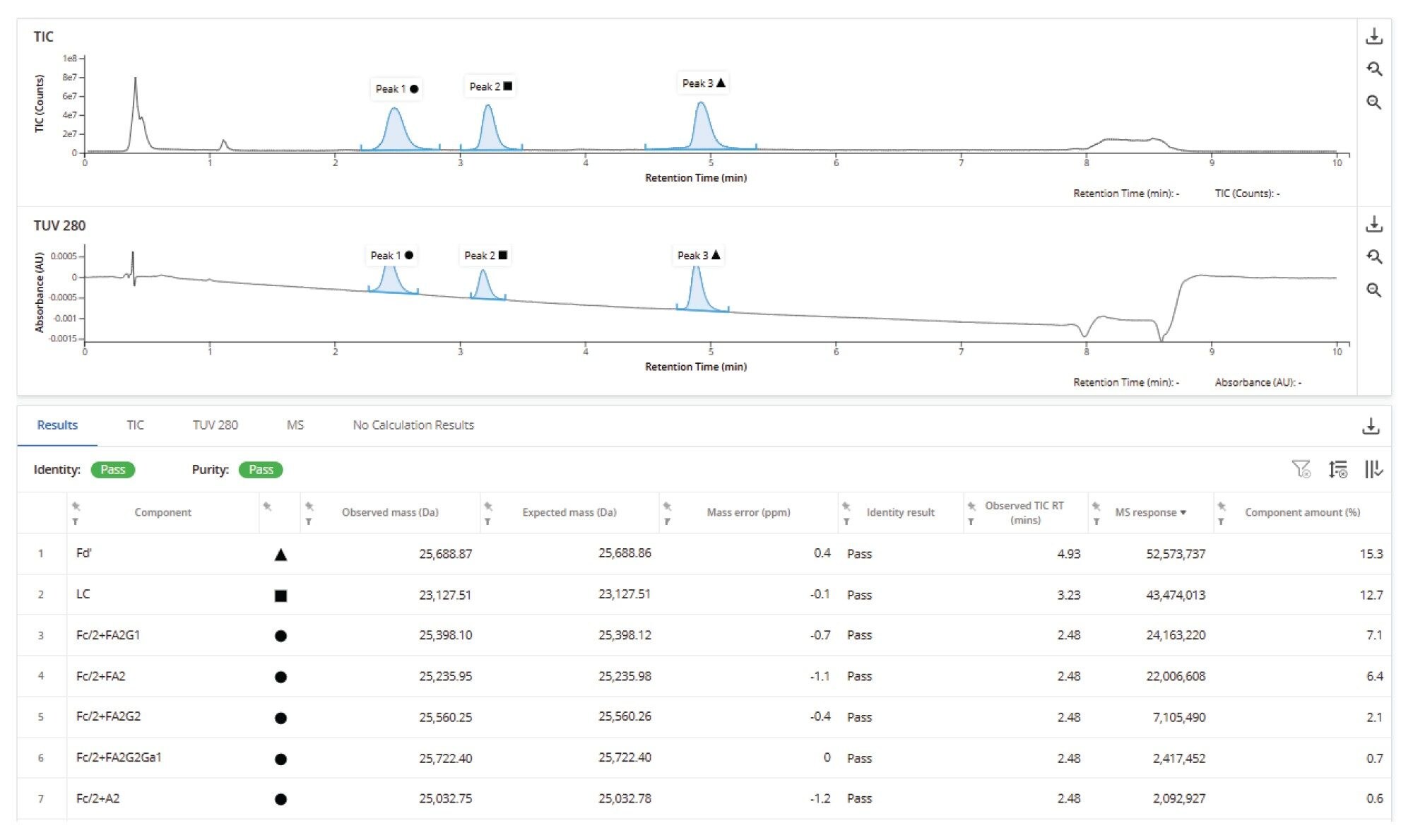
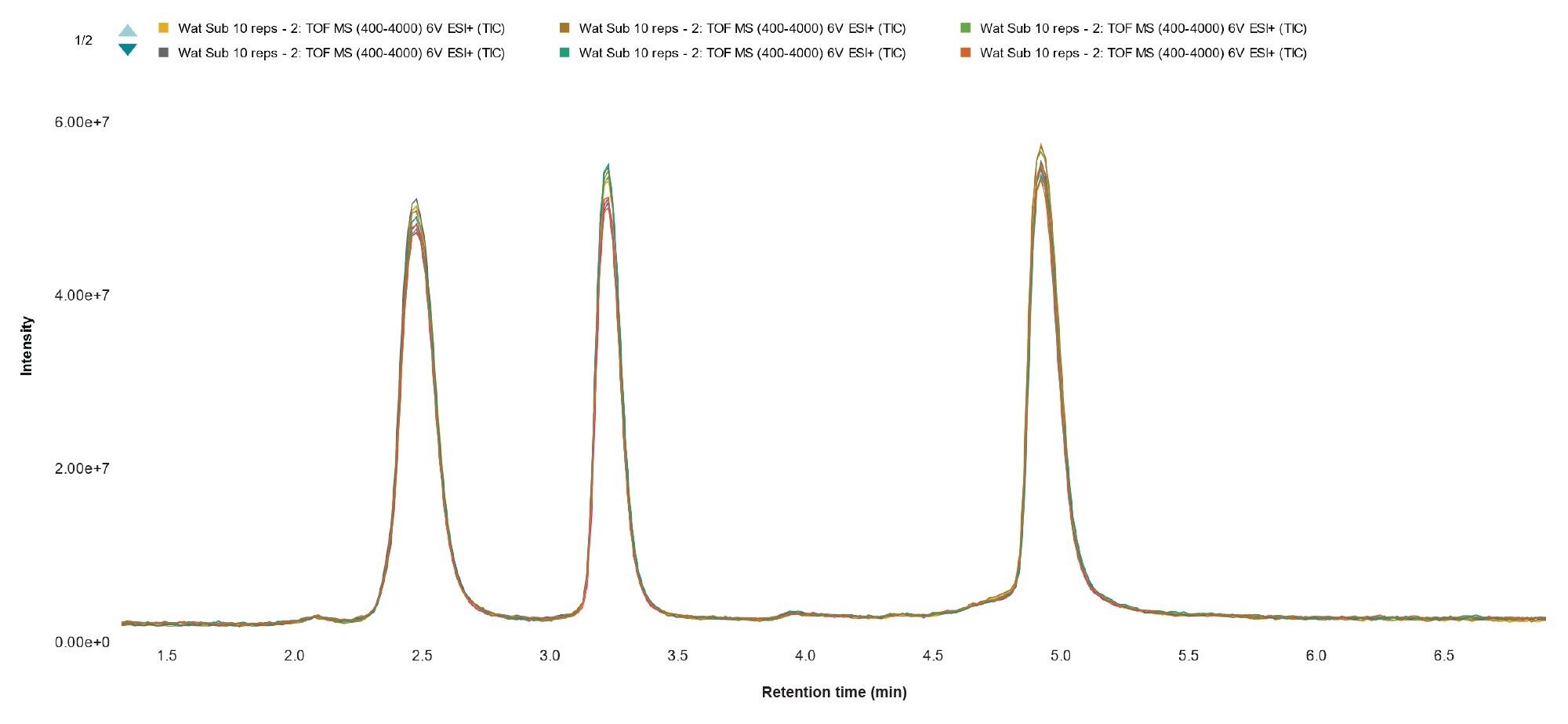
The chromatographic TIC response chromatograms from ten replicate subunit injections (Figure 7) reveal the overlap of consistent UPLC chromatography and consistent LC-MS detection response across the replicates. This is important not only for confident assessment of an individual sample, but for setting expectations of assay performance and supporting comparability studies from multiple batches or production processes.
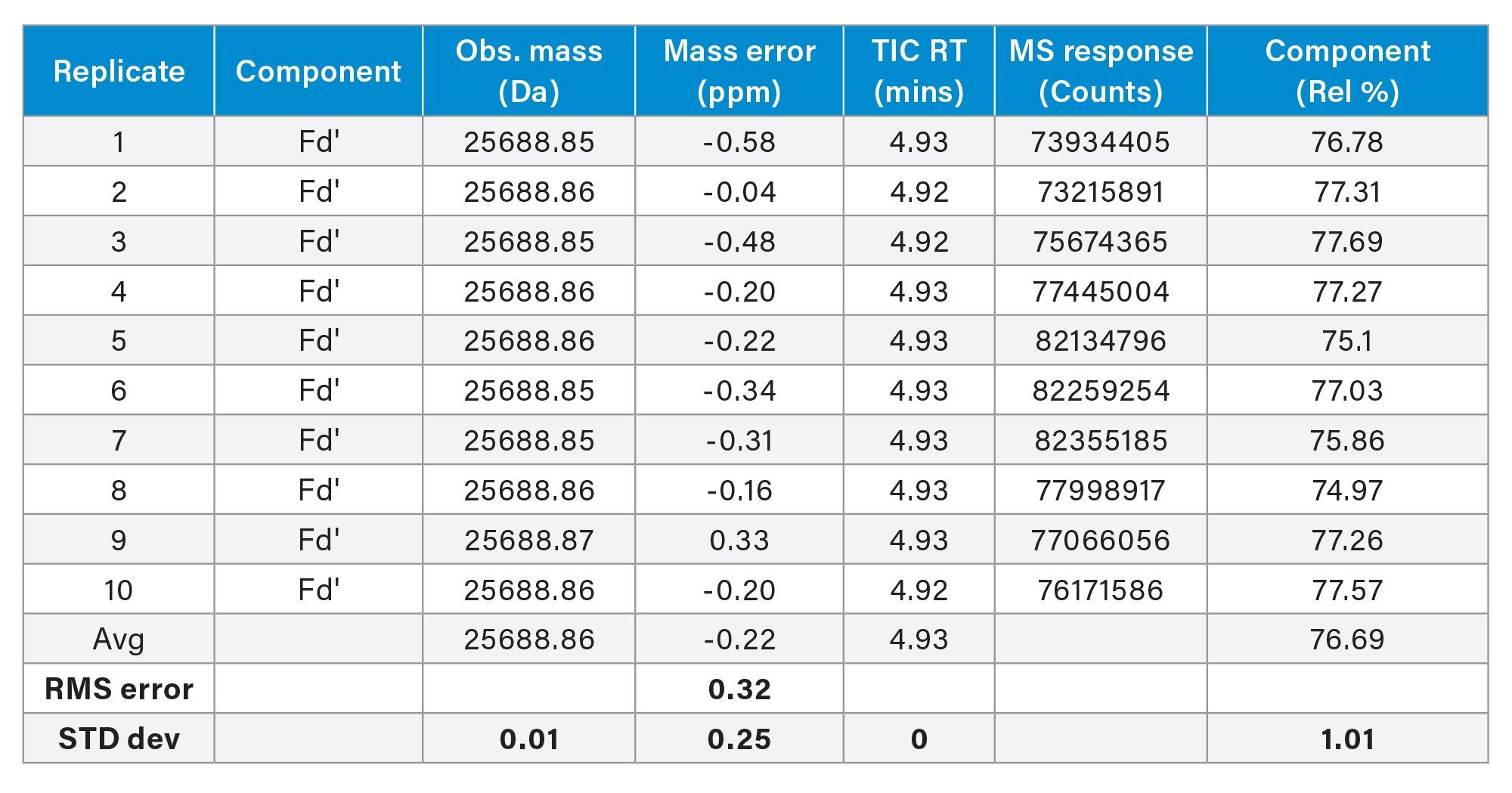
This consistency is also apparent in the analysis of data from the deconvoluted mas spectral results from the 25 kDa Fd' subunits, this time employing optimized deconvolution parameters (optimized elemental composition) to further refine the measured subunit mass. The results demonstrate a mean mass error of approximately 220 parts per billion (ppb), with a root mean square (RMS) error of 320 ppb (Figure 8). These findings underscore the high precision and reproducibility of the mass spectrometric measurements for this protein subunit under these simple assay conditions.
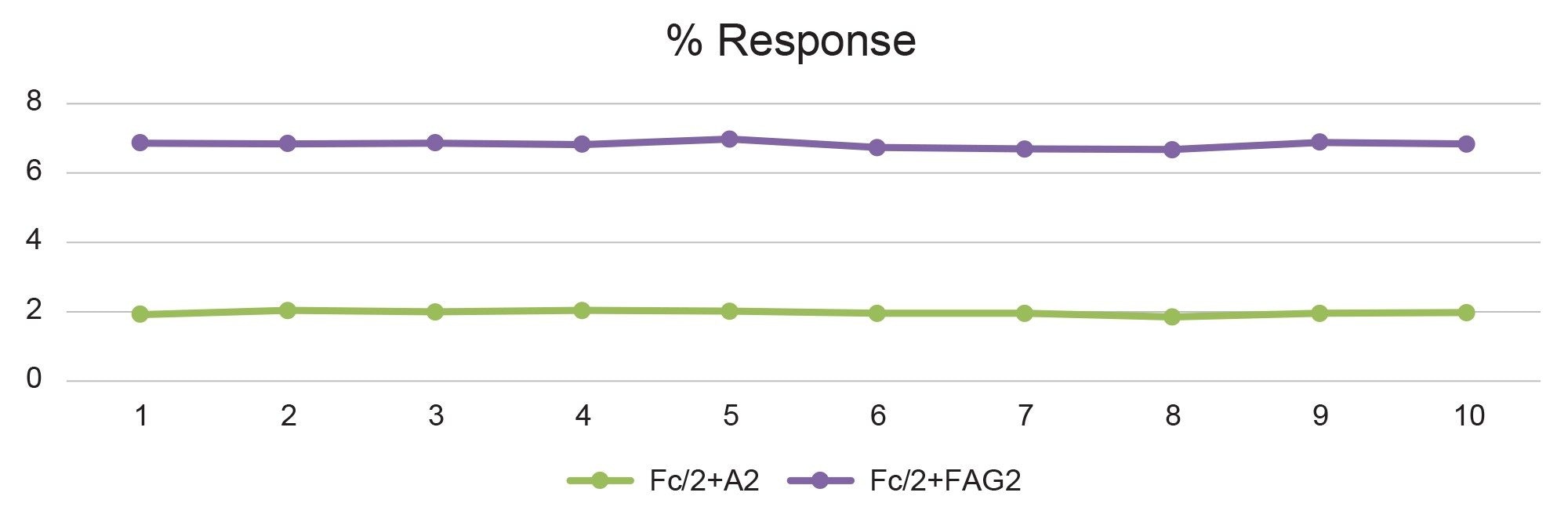
Finally, the deconvoluted mass spectral response data for two of the lower intensity glycoforms of the digested Fc subunit (Figure 9) similarly demonstrates the consistency of relative glycoform quantification over the ten replicate injections.
Subunit based analysis can provide great insight into the product variation of a monoclonal antibody, both for the initial characterization, and for ongoing analyses supporting product and process development. The capabilities of the Xevo MRT Mass Spectrometer for subunit based analysis include the ability to maintain consistent performance (resolution, mass accuracy) across a broad m/z range for subunit variant charge states and spanning multiple orders of magnitude of MS response. The system's high sensitivity and sub-to low-ppm mass accuracy enables the confident assignment of lower-abundance modifications, with the consistency of measurement to enable statistical validation of the performance of the method, and facilitate multi-batch quality and process development QbD studies.
This exceptional MS detection performance was complemented with a streamlined automated compliant-ready workflow, within the waters_connect INTACT Mass App that supported integrated data acquisition, processing, review, and reporting for subunit data acquired from individual samples, and summarized across a larger sample set.
Overall, mAb subunit based analysis on the Xevo MRT Mass Spectrometer System has been demonstrated to be a robust and efficient platform for enabling biopharmaceutical analysts to make confident decisions on biotherapeutic development and commercialization without the complications that can arise from manual data processing/reporting using spreadsheets and other third party software tools.
For more information, please visit https://www.waters.com/nextgen/global/products/mass-spectrometry/mass-spectrometry-systems/xevo-mrt.html
720008755, March 2025