Illustrating the Use of Cyclic Ion Mobility to Enhance Specificity for branched-PFAS Isomer Analysis
Solo para fines de investigación. No se debe utilizar para procedimientos de diagnóstico.
Abstract
The SELECT SERIES™ Cyclic™ Ion Mobility Mass Spectrometer has been used to analyse a series of per and polyfluorinated alkyl substances (PFAS) analytical standards comprised of structurally equivalent perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) branched isomers. Travelling wave ion mobility (TWIM) CCS values have been measured and show excellent agreement (Δ CCS 0.7%) when compared to drift tube and trapped ion CCS measurements.
PFOA produces characteristic isomer dimers, and measured CCS provide additional specificity. Branched isomer standard byproducts have been characterised using single component fragmentation spectra. Chromatographically coeluting isomers, are resolved using ion mobility enhanced peak capacity. Using the multipass capability of the Cyclic IMS to increase resolution from R~65 to R~145, resulted in a factor of eight improvement in peak-to-peak resolution (RsP-P).
This study demonstrates, how the benefits of LC-cIM-MS can be implemented to confidently identify known and unknown linear PFAS (L-PFAS) and branched PFAS (br-PFAS), enhancing specificity for environmental analysis and exposomics studies.
Benefits
- CCS values provide additional specificity to distinguish PFAS isomers
- LC-cIM-MS is a routine and robust non-targeted screening approach that provides enhanced peak capacity, using a combination of chromatographic, m/z and ion mobility resolution. The combined resolution enables differentiation of isobaric and isomeric analytes, including PFHxS, PFOS, and PFOA isomers
- Multipass cyclic IMS enhances peak capacity, allowing for the resolution of chromatographically coeluting isomers. This improvement enables single component fragment ion spectra to be obtained, providing greater certainty in the structural elucidation of both known and unknown isomeric polyfluoroalkyl substances
- Comparing Travelling wave ion mobility (TWIM) CCS values to drift tube and trapped ion CCS measurements (Δ CCS <0.7%), confirms CCS values can be used to reliably perform transferable PFAS analysis research
Introduction
Products incorporating PFAS, have evolved and are used extensively within society because of the unique properties imparted by the fluoroalkyl moiety. Adverse health conditions resulting from environmental exposure to PFAS have previously been described.1,2 Stringent guidelines and legislation have been introduced because of the associated toxicity resulting from PFAS exposure.3-7 Environmental fate and impact of alternative PFAS are being assessed, several studies indicate that new alternative PFAS have similar toxicity to banned PFAS.8
Although PFOA and PFOS are banned, production continues in developing countries. Electrochemical fluorination (ECF) and telomerization are the major manufacturing processes for PFOA. Rearrangement and breakage of the carbon chain takes place in the ECF process, leading to the production of approximately 78% linear and 22% branched isomers.9 Primarily PFOS is has been manufactured by ECF, with 70% linear and 30% branched isomers resulting. Over the past two decades there has been prevalent focus upon multiple PFAS in aquatic environments, comparatively, branched-PFAS (br-PFAS) isomers have received little attention, due to the incumbent analytical challenge, and low abundance as the root cause.10
Chemical properties of PFAS isomers can impact environmental fate, higher polarity branched isomers are more likely to remain in water, whereas L-PFAS are preferentially adsorbed/absorbed to soil/sediment. Impact upon environmental clean-up treatment efficiency is evidenced with a differential response obtained for L-PFAS and br-PFAS.10 Biotransformation rate is notably influenced by PFAS branching geometry and L-PFOS preferentially accumulates in animals, whereas preferential accumulation of br-PFAS in humans is evidenced by higher percentage of br-PFAS compared to ECF production L-PFAS/br-PFAS ratios.11
Often br-PFAS are analysed and reported as a sum of the branched components.3,4 This has challenges when conducting targeted analysis, as identification by ion ratio can be challenging as the product ion fragmentation pattern can be different between the linear PFAS component and the br-PFAS to which they are being compared to. There is also the inherent assumption that the br-PFAS have a similar/same ionisation potential to the L-PFAS that they are being quantified against. The third challenge is that the profile of the br-PFAS present in the analytical standard can be different and with targeted analysis using tandem quadrupole technology this cannot be determined.
The number of known and unknown emerging PFAS, PFAS precursors and transformation products are increasing. As a result, the use of non-targeted high resolution mass spectrometry (HRMS) strategies will continue to increase (~1000 unknown PFAS identified).12 These strategies will be employed alongside targeted analysis strategies. Non-targeted ultra-high performance liquid chromatography cyclic ion mobility (UHPLC-cIM) is a particularly amenable because of its ability to resolve and differentiate isomeric species, as well as providing enhanced resolution of thousands of analytes in a single acquisition.12
The ability to resolve isomers is of notable importance, it has previously been proposed that LC-cIM-MS is an analytical strategy that can be used to potentially cross correlate PFOS isomer structure and toxicity.13 The need to exploit the benefits if LC-cIM-MS to improve understanding of the environmental impact of PFAS exposure resulting from isomeric distribution, is emphasised, given that L-PFAS and br-PFAS have been shown to have specific detrimental health association and infant burden during pregnancy.14,15
Herein we describe LC-cIM-MS analyses performed using a SELECT SERIES Cyclic IMS system (see Figure 1), where enhanced peak capacity Pc, and increased peak-to-peak resolution RsP-P is obtained. Chromatographically coeluting PFOS isomers are resolved and structural elucidation performed to identify br-PFOS byproducts, based on the single component isomeric fragmentation spectra obtained.
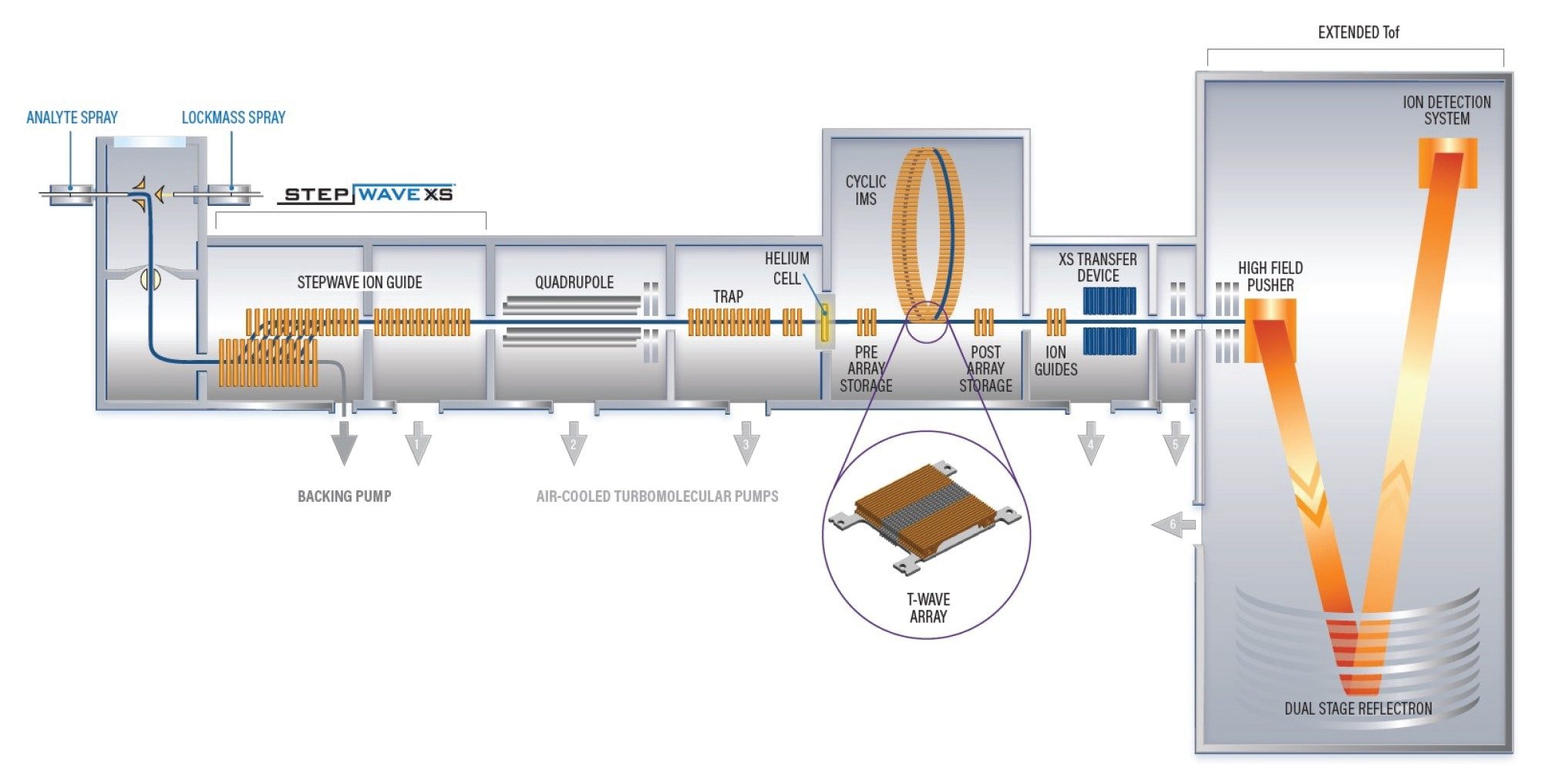
Experimental
Sample Description
Native PFAS Solution/mixtures: PFAC30PAR, br-PFOSK, br-PFOAK, br-PFHxSK (Wellington Laboratories).
LC Conditions
|
LC system: |
ACQUITY™ Premier System modified with PFAS Kit and Atlantis™ Premier BEH™ C18 AX Isolator Column, 2.1 x 50 mm, 5 µm (p/n: 186010926).16 |
|
Column: |
ACQUITY UPLC™ BEH™ C18 Column (100 mm x 2.1 mm, 1.8 µm) |
|
Column temperature: |
35 °C |
|
Sample temperature: |
6 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.3 mL/min |
|
Mobile phase A: |
95 H2O (2 mM ammonium acetate):5 MeOH |
|
Mobile phase B: |
MeOH (2 mM ammonium acetate) |
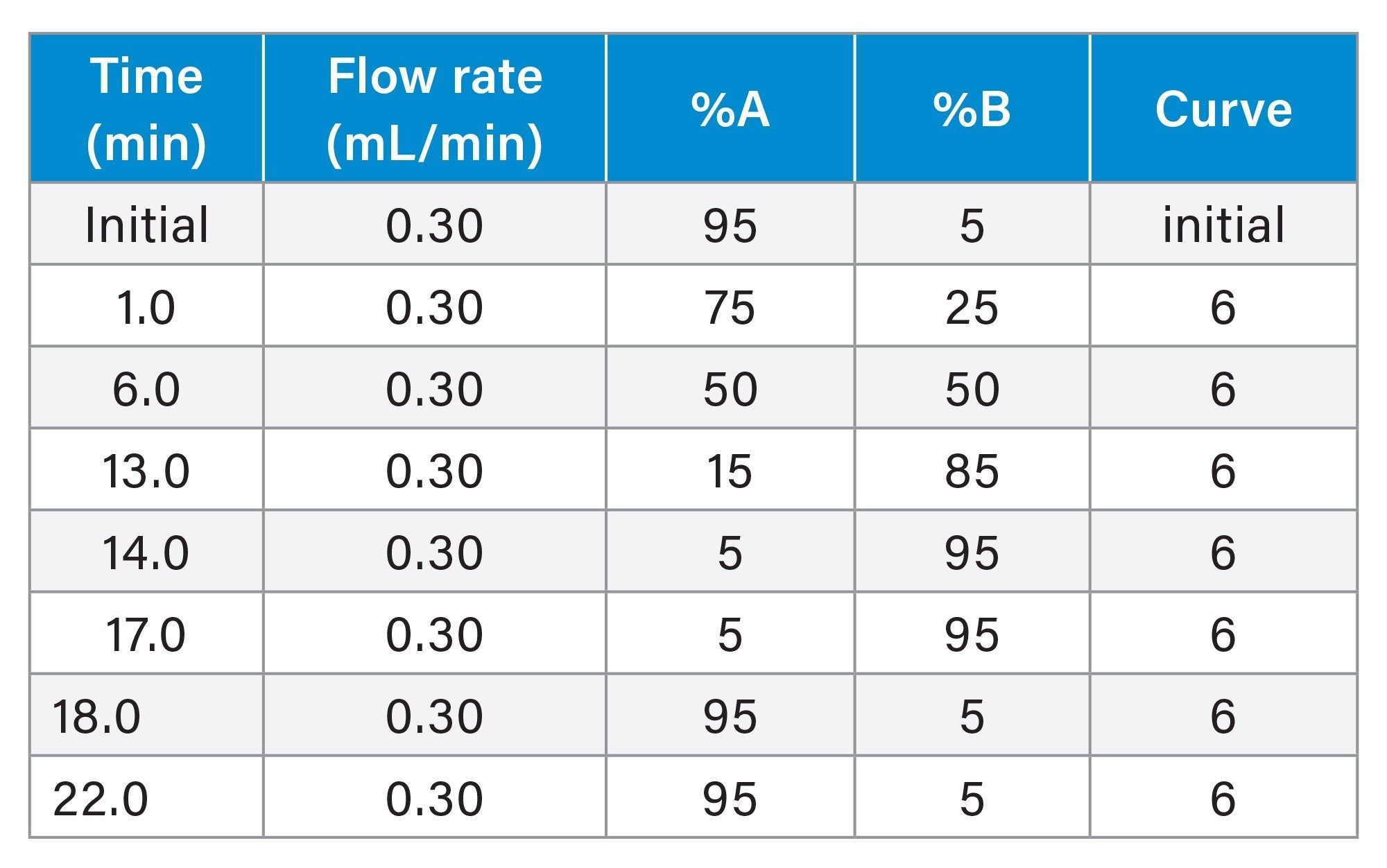
Gradient Table
MS Conditions
|
Ionization mode: |
ES- |
|
Acquisition range: |
m/z 50–1200 (HDMSE) and m/z 499(HDMS/MS) |
|
Capillary voltage: |
0.5 kV |
|
Collision energy: |
HDMSE (CE 20–70 Ev), HDMS/MS (CE 6eV) |
|
Cone voltage: |
10 V |
|
Desolvation temperature: |
350 ºC |
|
Source temperature: |
100 ºC |
|
Acquisition rate: |
0.16 seconds (HDMSE) R~65 0.15 seconds HDMS/MS R~145 |
Data Management
|
Chromatography and MS: |
MassLynx™ 4.2 SCN1026 Software |
|
Informatics: |
waters_connect™ 3.1.0.243 Software |
Results and Discussion
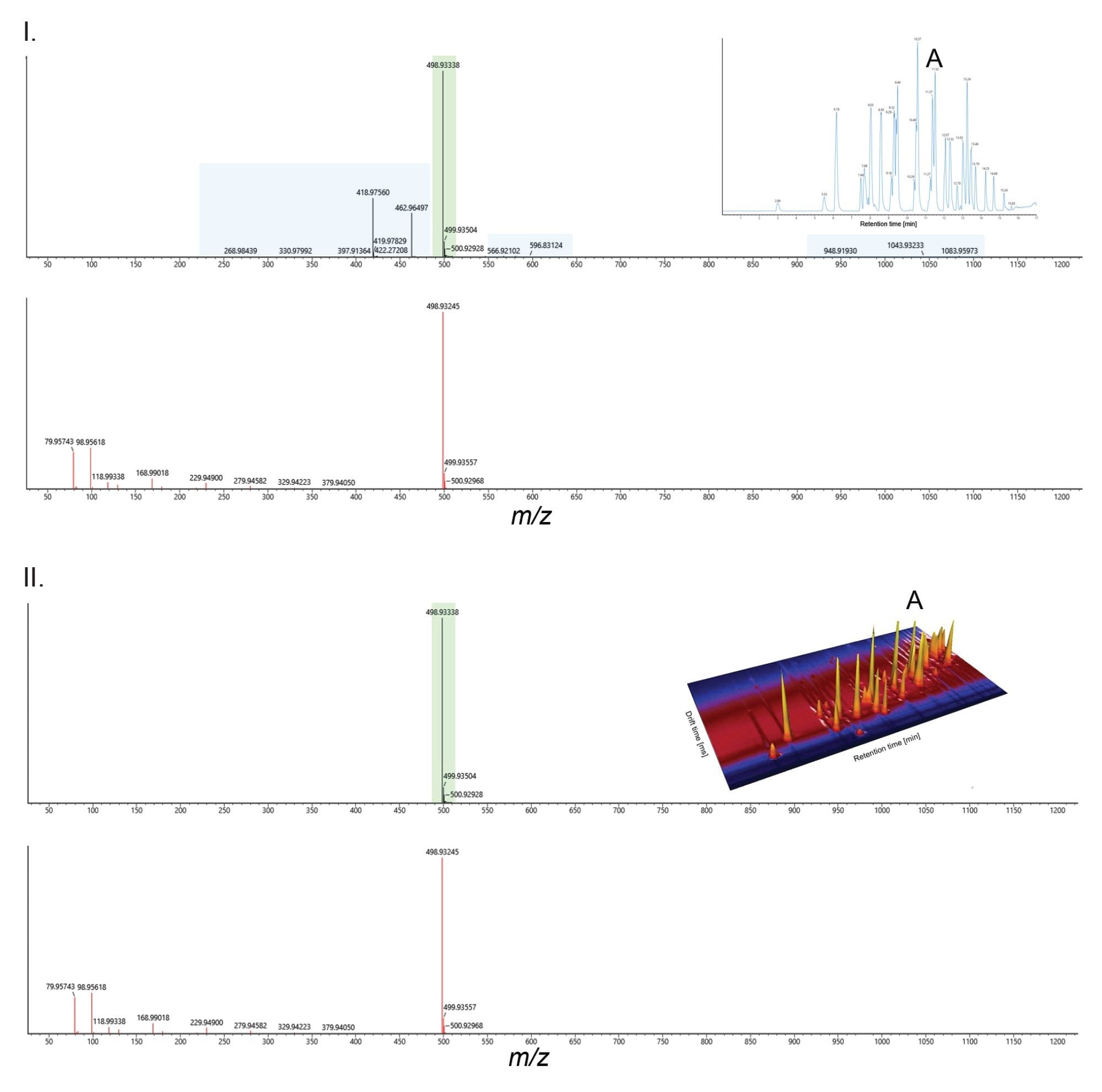
Peak capacity (Pc) can be used to represent the maximum number of resolved peaks in a separation window e.g. chromatogram or ion mobility spectrum. Using IM, a factor of 3 to 10 increase in peak capacity can be achieved, depending on IM resolution, m/z resolution and sample complexity.17,18 A single pass of the cyclic IMS device on the SELECT SERIES Cyclic IMS mass spectrometer provides an IM resolution of R~65. Using IM as a second dimension of separation with UHPLC helps clean up spectra by resolving coeluting analytes (e.g., peak A at 11.52 minutes, Figure 2). This approach addresses sample complexity and enhances specificity in data-independent acquisitions.
Resolving isomers is a greater challenge, br-PFOS isomers can be subtly differentiated (see Figure 3). The apex of the arrival time distributions (ATDs)determined for the mix of br-PFOS isomers, are differentiated by ~0.3 ms. The apex of the ATD’s of perflouro-5-methyl heptane sulphonic acid (P5MHpS) perflouro-6-methyl heptane sulphonic acid (P6MHpS) are differentiated by ~0.1 ms. Increasing IM separation can enhance the resultant Pc, enabling more specific precursor fragment alignment to facilitate the attainment of single component ion mobility product ions and PFOS isomer differentiation. This is important because collision induced dissociation (CID) of PFOS isomers produces isomeric product ions, making it hard to confidently identify structures from a composite spectrum of coeluting PFOS isomers.
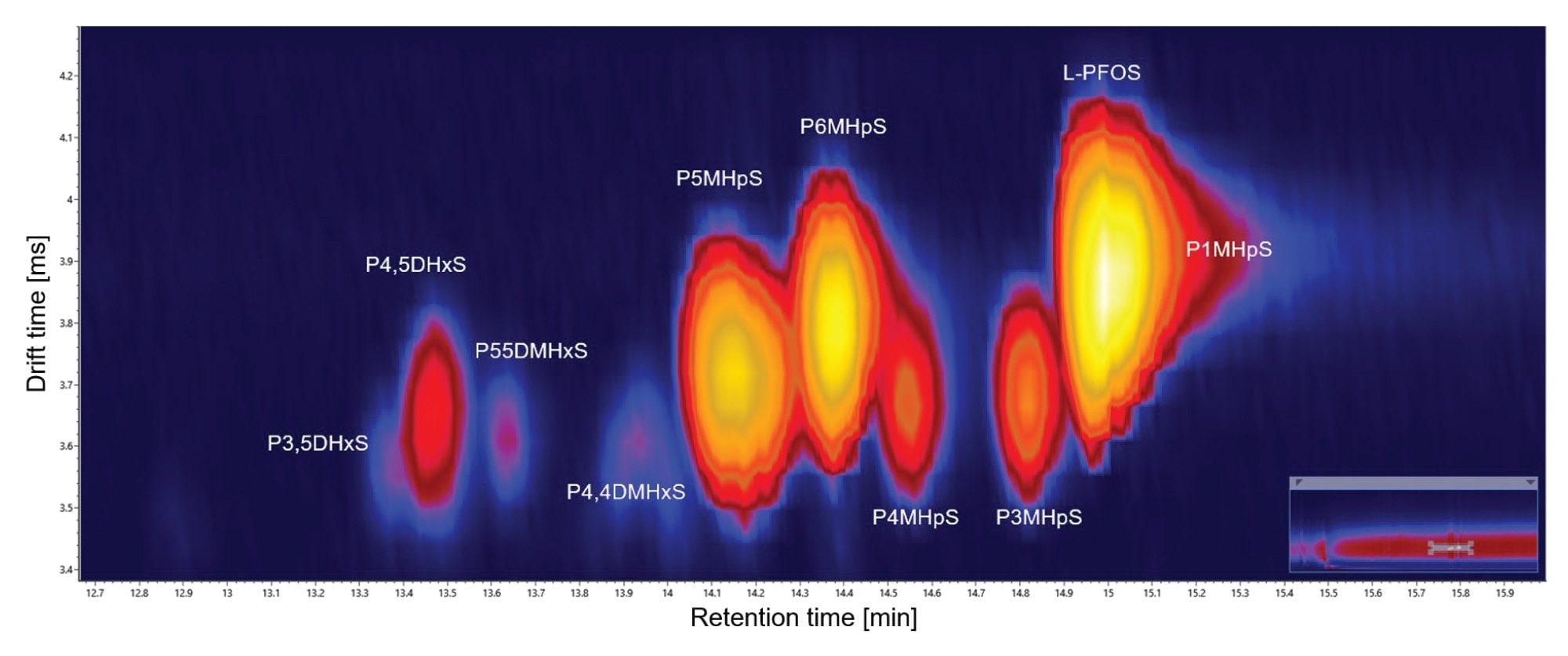
To further resolve PFOS isomers, peak capacity can be improved using multi pass IMS. This is illustrated in Figure 4, for the analysis of perflouro-1-methyl heptane sulphonic acid (P1MHpS), where two chromatographically coeluting PFOS byproduct isomers have been identified as P5MHpS and P6MHpS. Using IM resolution of R~65, Rsp-p=0.1 is achieved. However, IM resolution of R~145 provides an 8-fold improvement (Rsp-p=0.8) and resolution of P5MHpS and P6MHpS is achieved. This enables the single component ion mobility product ion spectrum of each unknown PFOS isomer to be determined and confident structural elucidation of each respective br-PFOS isomer (see Figure 5).
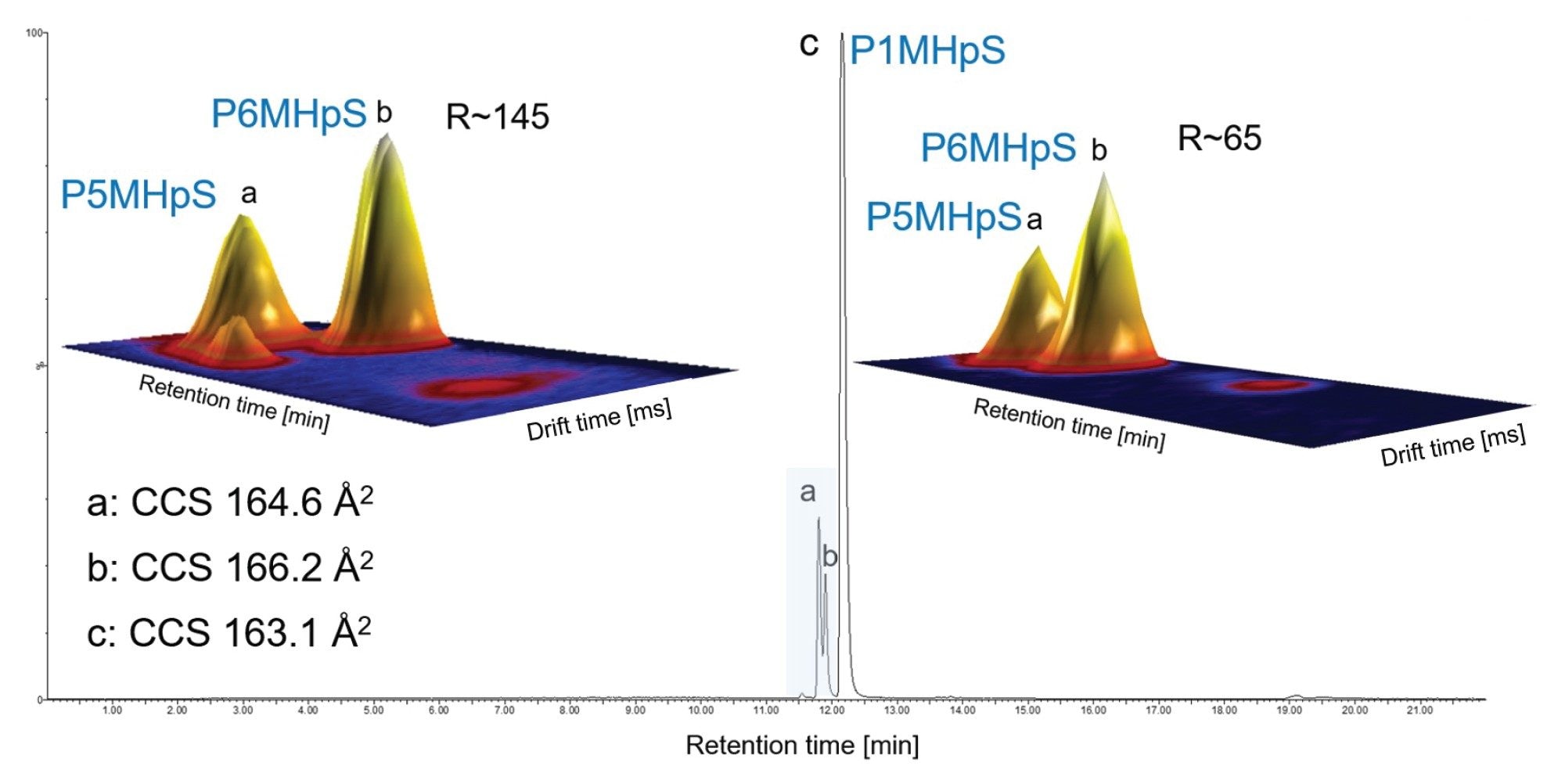
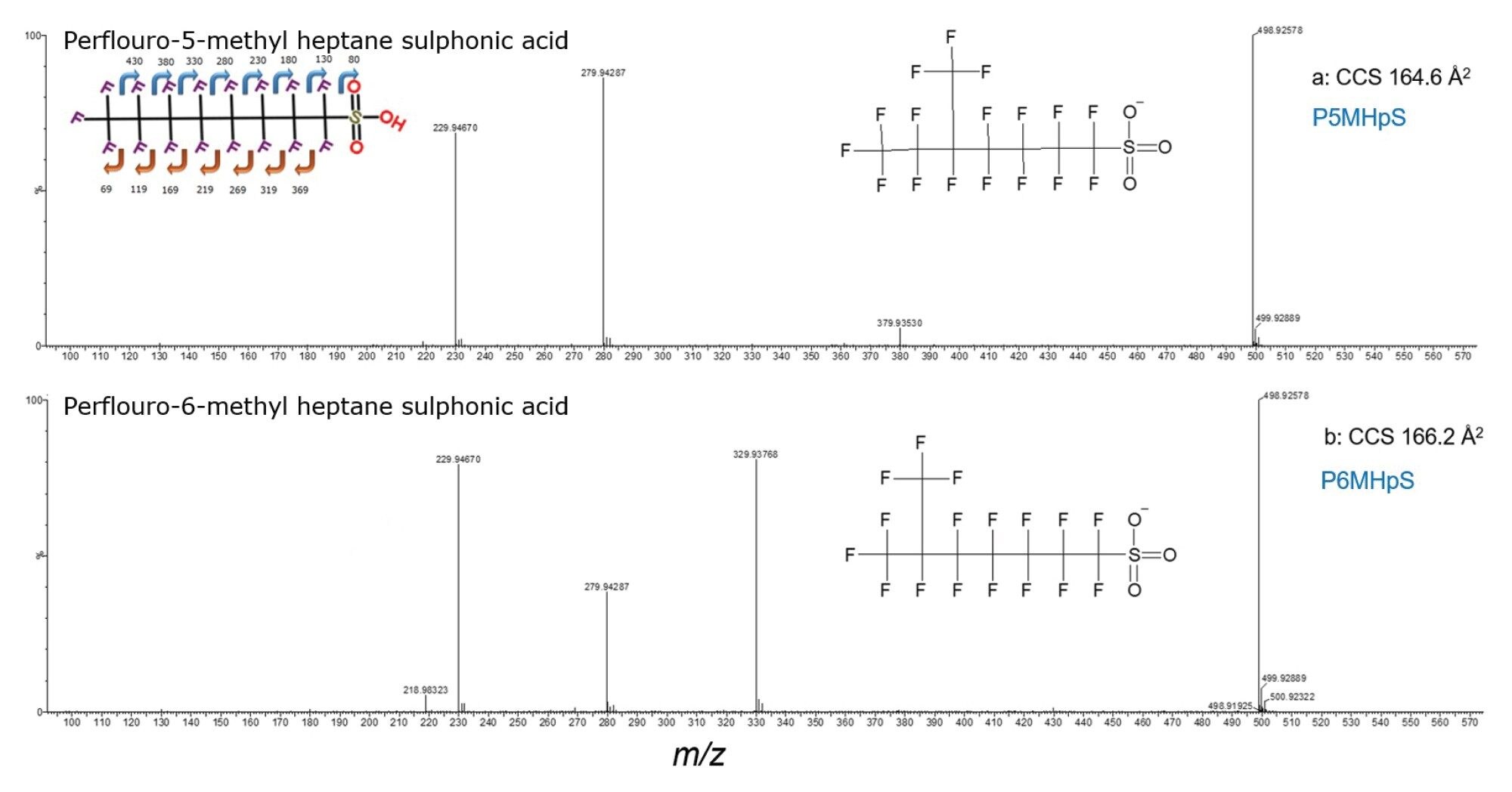
CCS values provide an additional identification descriptor which add confidence to assignments. This is particularly useful when analytes are present at low ion intensities where the mass spectra may exhibit insufficient characteristic product ion information or may be weak or unobserved. CCS values of L-PFOS and br-PFOS isomers have been characterised, enabling identification of individual PFOS isomers that may be used to identify PFOS byproducts (see Table 1). A comparison to published drift tube DT CCS values is shown, where an average Δ CCS 0.31% has been obtained and 0.52% compared to trapped IM.19 For the branched isomer byproducts identified in the 1MHpS standard, P5MHpS Δ CCS (0.67%) and P6MHpS Δ CCS (0.48%) were obtained compared to library values (Table 1). The br-PFOA isomer CCS values have a range between 149.8 Å2 and 154.7 Å2. Additionally, br-PFOA isomer dimers CCS values have been characterized providing additional CCS fingerprint specificity (see Table 2). A mixture of br-PFHxS isomers have also been analysed, CCS values assignment was facilitated using the observed isomer intensity profile and certificate of analysis isomer percentage composition (see Table 3). The br-PFHxS isomers values have a range between 149.1 Å2 and 144 Å2. For L-PFHxS a CCS value of 149.1 Å2 was obtained (Δ CCS (0.67%) compared to literature).19 The excellent agreement of CCS values obtained on different platforms, supports the utility of CCS values as an identification descriptor that enhances specificity and efficiency in an inevitable increasing number of required PFAS analyses.
![br-PFOS isomer [M-H]- TWIM CCS values and comparative DT CCS values](/content/dam/waters/en/app-notes/2025/720008723/720008723en-t1.jpg.82.resize/img.jpg)
![br-PFOA isomer [M-H]- and dimer CCS values](/content/dam/waters/en/app-notes/2025/720008723/720008723en-t2.jpg.82.resize/img.jpg)
![br- PFHxS [M-H]- CCS values](/content/dam/waters/en/app-notes/2025/720008723/720008723en-t3.jpg.82.resize/img.jpg)
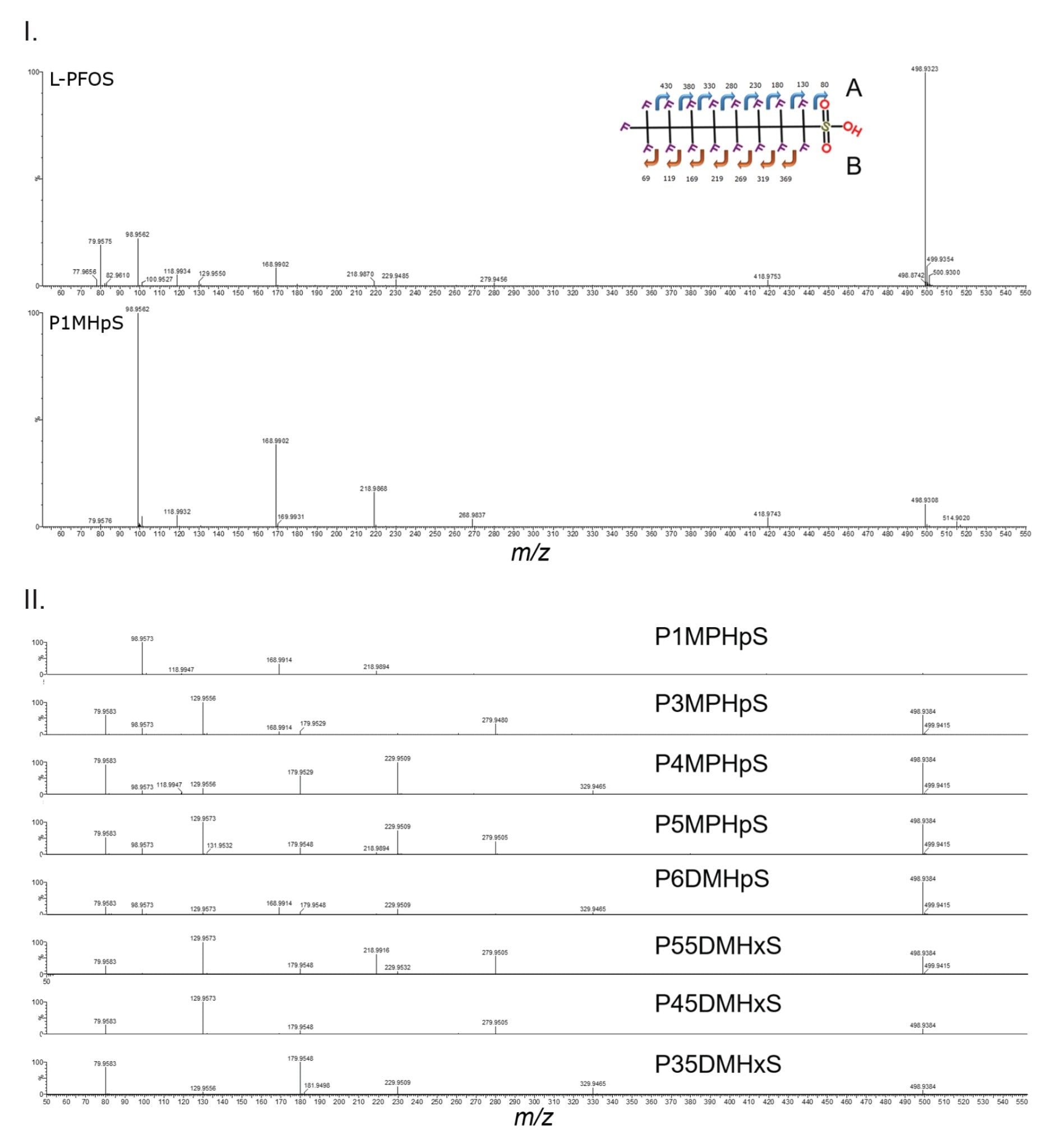
In the case of L-PFOS and P1MHpS equivalent retention times are observed, however the isomers are differentiated by their CCS values (>3 Å2) and observed ion mobility product ion spectra. The L-PFOS anion fragmentation pathway produces both a SO3- m/z 80 and SO3F- m/z 99 fragment ions of approximate equal abundance. In Figure 6 (I), L-PFOS and P1MHpS are presented, the fragment ion spectrum of L-PFOS is comprised of fragment ions resulting from fragmentation pathway a and b, whereas P1MHpS fragment ions are produced solely from fragmentation pathway b. For P1MHpS the m/z 99 forms the base peak of the of the product ion spectrum and m/z 80 is approximately 1%. br-PFOS isomer fragmentation spectra have been acquired using a fixed collision energy (Figure 6 (II)), characteristic isomer fragmentation spectra are comprised of major and minor isomeric fragment ions. Using ion mobility spectral clean up aids visualisation of critical fragmentation information to facilitate isomer structural elucidation. Although illustrated for standards, the benefits of this approach can be adopted for identification of both known and unknown PFAS.13
Conclusion
The implementation of HRMS for PFAS analysis has led to the identification of previously unknown PFAS. However, the focus has primarily been on linear PFAS forms, neglecting their br-PFAS isomer byproducts. LC-IM-MS offers a solution by enabling non-targeted screening to detect and identify known, unknown, and emerging PFAS, including transformation products, and crucially, differentiate br-PFAS byproducts. This technique combines three dimensions of resolution—retention time, m/z, and CCS specificity.
Characteristic CCS values for br-PFOS, br-PFOA, and br-PFHxS isomers have been determined and shown to be independent of IM technology (Δ CCS <0.7%). LC-IM-MS has resolved chromatographically coeluting br-PFOS isomers, generating single-component characteristic isomeric product ion spectra for confident structural elucidation. Even at low analyte concentrations when the absence of product ion spectra is problematic, L-PFAS and br-PFAS CCS fingerprints can be correlated with PFAS product-specific fingerprints to facilitate environmental source tracking.
With growing global concerns about environmental exposure to PFAS, the demand for PFAS sample analyses will continue to grow. This study demonstrates that LC-cIM-MS can play a critical role in analytical strategies, particularly in correlating the genotoxicity and environmental fate of br-PFAS.
References
- Enhanced Identification Confidence and Specificity for PFAS Analysis Using Cyclic Ion Mobility Mass Spectrometry Collision Cross Sections, Waters Application Note, 720008536, 2024.
- Kirkwood-Donelson KI, Dodds JN, Schnetzer A, Hall N, Baker ES. Uncovering per- and polyfluoroalkyl substances (PFAS) with nontargeted ion mobility spectrometry–mass spectrometry analyses. Sci. Adv.9, eadj7048(2023).
- https://www.epa.gov/system/files/documents/2024-01/method-1633-final-for-web-posting.pdf.
- https://eurl-pops.eu/working-groups#_pfas
- https://cdn.dwi.gov.uk/wp-content/uploads/2021/10/04203217/Information-Letter-PFAS-Monitoring.pdf
- https://www.maine.gov/dep/spills/topics/pfas/PFAS-products/
- https://www.assemblee-nationale.fr/dyn/16/textes/l16t0276_texte-adopte-provisoire.pdf
- Conley JM, Lambright CS, Evans N, McCord J, Strynar MJ, Hill D, Medlock-Kakaley E, Wilson SV, Gray Jr LE. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ Int. 2021 Jan;146:106204.
- Benskin JP, Yeung LW, Yamashita N, Taniyasu S, Lam PK, Martin JW. Perfluorinated acid isomer profiling in water and quantitative assessment of manufacturing source. Environ Sci Technol. 2010 Dec 1;44(23):9049–54.
- Londhe K, Lee CS, McDonough CA, Venkatesan AK. The Need for Testing Isomer Profiles of Perfluoroalkyl Substances to Evaluate Treatment Processes. Environ Sci Technol. 2022 Nov 15;56(22):15207–15219.
- Chen X, Zhu L, Pan X, Fang S, Zhang Y, Yang L. Isomeric specific partitioning behaviors of perfluoroalkyl substances in water dissolved phase, suspended particulate matters and sediments in Liao River Basin and Taihu Lake, China. Water Res. 2015 Sep 1;80:235–44.
- Pavlovic R, Draghi S, Pellegrini A, Fornesi Silva C, Di Cesare F, Curone G, Arioli F, Fidani M. High-Resolution Mass Spectrometry Non-Targeted Detection of Per- and Polyfluoroalkyl Substances in Roe Deer (Capreolus capreolus). Molecules. 2024 Jan 27;29(3):617.
- McCullagh M, Lioupi A, Theodoridis G, Plumb R, Wilson I and Adams S. Using the Combined Peak Capacity of Liquid Chromatography and Cyclic Ion Mobility Mass Spectrometry to Enhance Analysis Efficiency and Specificity in PFAS Analysis. Waters Application Note, 720008671, 2024.
- Schulz K, Silva MR, Klaper R. Distribution and effects of branched versus linear isomers of PFOA, PFOS, and PFHxS: A review of recent literature. Sci Total Environ. 2020 Sep 1;733:139186.
- Varsi K, Huber S, Averina M, Brox J, Bjørke-Monsen AL. Quantitation of linear and branched perfluoroalkane sulfonic acids (PFSAs) in women and infants during pregnancy and lactation. Environ Int. 2022 Feb;160:107065.
- PFAS Analysis Kit for ACQUITY UPLC Systems, Waters User Guide, 720006689, 2019.
- Dwivedi P, Puzon G, Tam M, Langlais D, Jackson S, Kaplan K, J Mass Spectrom., 2010,45(12), 1383–1393.
- Grabarics M, Lettow M, Kirk AT, von Helden G, Causon TJ, Pagel K. Plate-height model of ion mobility-mass spectrometry: Part 2—Peak-to-peak resolution and peak capacity. J Sep Sci. 2021 Jul;44(14):2798–2813.
- Dodds JN, Hopkins ZR, Knappe DRU, Baker ES. Rapid Characterization of Per- and Polyfluoroalkyl Substances (PFAS) by Ion Mobility Spectrometry-Mass Spectrometry (IMS-MS). Anal Chem. 2020 Mar 17;92(6):4427–4435.
Waters, SELECT SERIES, Cyclic, ACQUITY, UPLC, BEH, waters_connect, Atlantis and MassLynx are trademarks of Waters Technologies Corporation. All other trademarks are the property of their respective owners.
720008723, March 2025